topic 6.2: Stratospheric Ozone
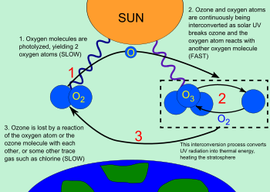 image from http://en.wikipedia.org/wiki/Ozone_layer
image from http://en.wikipedia.org/wiki/Ozone_layer
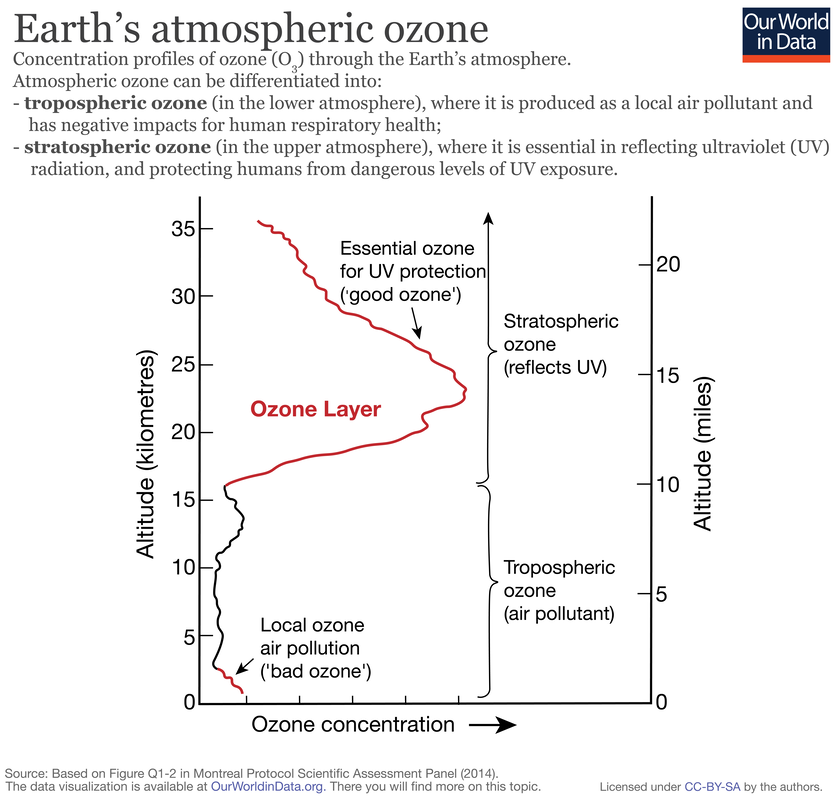
The ozone layer is the part of the Earth's atmosphere which contains relatively high concentrations of ozone (O3). "Relatively high" means a few parts per million - much higher than the concentrations in the lower atmosphere but still small compared to the main components of the atmosphere. Although the concentration of ozone in the ozone layer is very small, it is vitally important to life because it absorbs biologically harmful ultraviolet (UV) radiation from the Sun. The "thickness" of the ozone layer - that is, the total amount of ozone in a column overhead - varies by a large factor worldwide, being in general smaller near the equator and larger as one moves towards the poles. It also varies with season, being in general thicker during the spring and thinner during the autumn.
In this unit unit we will look at how human activities have resulted in the depletion of the ozone layer and the roles of government and non-government agencies in controlling the restricting ozone depleting substances.
This unit is a minimum of 2.5 hours.
In this unit unit we will look at how human activities have resulted in the depletion of the ozone layer and the roles of government and non-government agencies in controlling the restricting ozone depleting substances.
This unit is a minimum of 2.5 hours.
Significant Ideas:
- Stratospheric ozone is a key component of the atmospheric system because it protects living systems from the negative effects of ultraviolet radiation from the Sun.
- Human activities have disturbed the dynamic equilibrium of stratospheric ozone formation.
- Pollution management strategies are being employed to conserve stratospheric ozone.
Big questions:
- To what extent have the solutions emerging form this topic been directed at preventing environmental impacts, limiting the extent of the environmental impacts or restoring systems in which environmental impacts have already occurred?
- How are the issues addressed in this topic of relevance to sustainability or sustainable development?
- In what ways might the solutions explored in this topic after your predictions for the state of human societies and the biosphere some decades from now?
- Why has the Montreal Protocol been so successful? Will it continue to be successful?
- Outline the links between stratospheric ozone and sustainability/sustainable development.
- What are the greatest threats to stratospheric ozone likely to be, and from where, in the decades to come?
Knowledge and Understanding
6.2.U1 Some ultraviolet radiation from the Sun is absorbed by stratospheric ozone causing the ozone molecule to break apart. Under normal conditions the ozone molecule will reform. This ozone destruction and reformation is an example of a dynamic equilibrium.
[The use of chemical symbols, formulae or equations for the destruction of ozone is not required.]
[The use of chemical symbols, formulae or equations for the destruction of ozone is not required.]
- Outline how in the absence of pollution, stratospheric ozone provides an example of a dynamic equilibrium.
- Explain why seasonal changes occur in stratospheric ozone over the Polar Regions
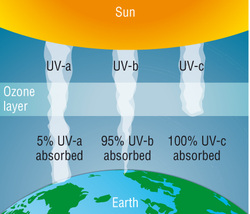 image from www.chemhume.co.uk
image from www.chemhume.co.uk
Ozone plays a key role in the temperature structure of the Earth's atmosphere. Without the filtering action of the ozone layer, more of the Sun's UV-B radiation would penetrate the atmosphere and would reach the Earth's surface. Ultraviolet radiation is absorbed during the formation and destruction of ozone from oxygen. You do not need to memorise chemical equations is not required.
Stratospheric ozone is formed naturally by chemical reactions involving solar ultraviolet radiation (sunlight) and oxygen molecules, which make up 21% of the atmosphere.
- solar ultraviolet radiation breaks apart one oxygen molecule (O2) to produce two oxygen atoms (2 O)
- each of these highly reactive atoms combines with an oxygen molecule to produce an ozone molecule (O3).
- these reactions occur continually whenever solar ultraviolet radiation is present in the stratosphere. As a result, the largest ozone production occurs in the tropical stratosphere
The ozone hole doesn’t exist year round: it’s seasonal. During the Antarctic winter, when polar stratospheric clouds become widespread, chemical reactions convert less reactive forms of chlorine into large amounts of highly reactive forms. These highly reactive gases have such a weak hold on their chlorine atoms that they are only stable in the dark.
When the Sun rises at the end of Southern Hemisphere winter, sunlight degrades these reactive gases, releasing “free radicals” of chlorine. A single chlorine-containing free radical can catalyze the destruction of thousands and thousands of ozone molecules. Ozone destruction usually peaks in mid-October. Ozone loss tapers off in late spring as the polar vortex weakens. Temperatures rise, and fewer clouds form. Ozone-rich air from lower latitudes mixes back into the polar stratosphere, and the ozone hole disappears until the next spring
When the Sun rises at the end of Southern Hemisphere winter, sunlight degrades these reactive gases, releasing “free radicals” of chlorine. A single chlorine-containing free radical can catalyze the destruction of thousands and thousands of ozone molecules. Ozone destruction usually peaks in mid-October. Ozone loss tapers off in late spring as the polar vortex weakens. Temperatures rise, and fewer clouds form. Ozone-rich air from lower latitudes mixes back into the polar stratosphere, and the ozone hole disappears until the next spring
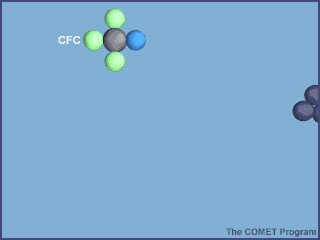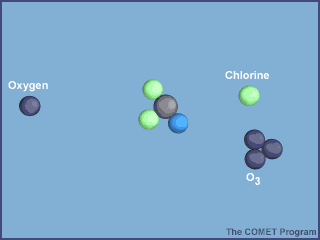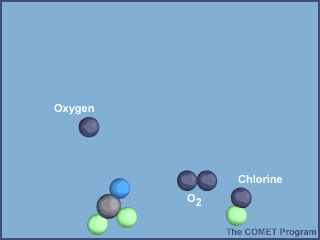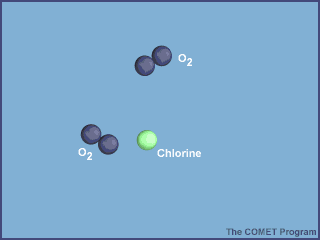
6.2.U2 Ozone depleting substances (including halogenated organic gases such as chlorofluorocarbons—CFCs) are used in aerosols, gas-blown plastics, pesticides, flame retardants and refrigerants. Halogen atoms (such as chlorine) from these pollutants increase destruction of ozone in a repetitive cycle, allowing more ultraviolet radiation to reach the Earth.
[The use of chemical symbols, formulae or equations for the destruction of ozone is not required.]
[The use of chemical symbols, formulae or equations for the destruction of ozone is not required.]
- Discuss the threats to stratospheric ozone.
- Identify the units used to measure stratospheric ozone.
Halogenated organic gases are very stable under normal conditions but can liberate halogen atoms when exposed to ultraviolet radiation in the stratosphere. These atoms react with monatomic oxygen and slow the rate of ozone
re‑formation. Pollutants enhance the destruction of ozone, thereby disturbing the equilibrium of the ozone production system
Ozone-depleting substances (ODS)
How ozone is depleted by CFC’s:
re‑formation. Pollutants enhance the destruction of ozone, thereby disturbing the equilibrium of the ozone production system
Ozone-depleting substances (ODS)
- Chlorofluorocarbons (CFCs or freons) - propellants in spray cans, foam, refrigerants
- Hydrochlorofluorocarbons (HCFCs) - replacements for CFCs
- Halon - fire extinguishers
- Methyl bromide - pesticides
- Nitrogen oxides - bacterial breakdown of nitrites and nitrates in the soil, supersonic aircraft
- Carbon tetrachloride - cleaning substance
How ozone is depleted by CFC’s:
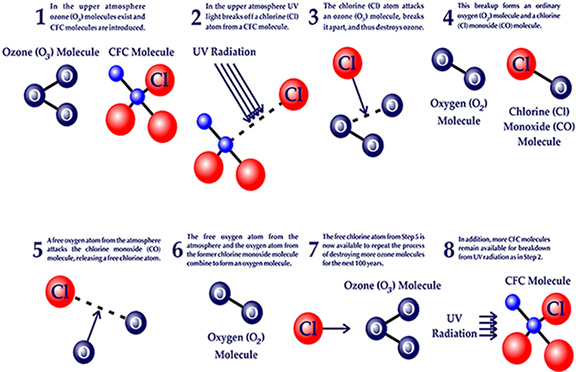
- UV radiation breaks off a chlorine atom from a CFC molecule.
- The chlorine atom attacks an ozone molecule (O3), breaking it apart and destroying the ozone.
- The result is an ordinary oxygen molecule (O2) and a chlorine monoxide molecule (ClO).
- The chlorine monoxide molecule (ClO) is attacked by a free oxygen atom releasing the chlorine atom and forming an ordinary oxygen molecule (O2).
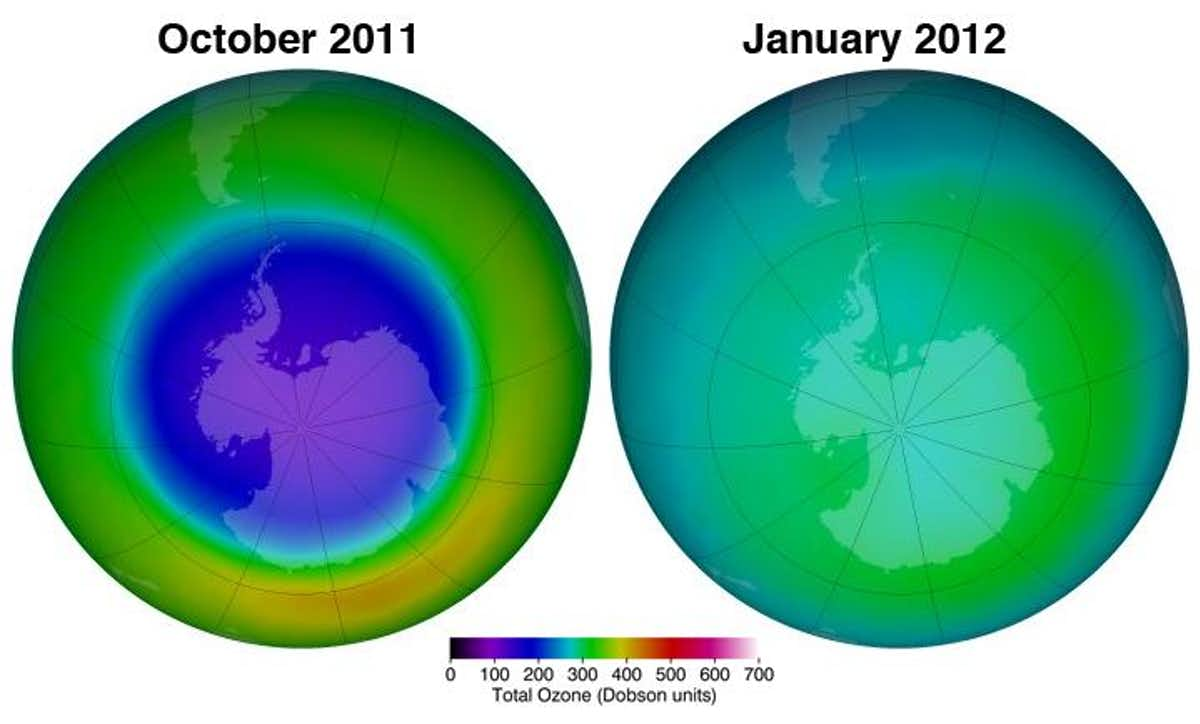
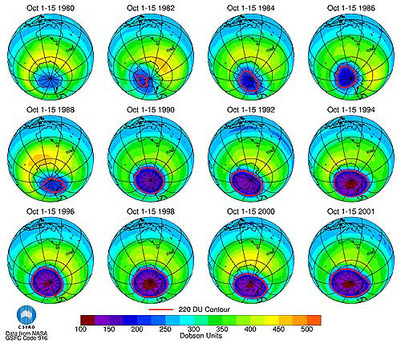
The total amount of ozone that constitutes the atmosphere is measured in Dobson Units (DU), which is a value determine by measuring the concentration of ozone molecules in a column of air that extends from the Earth’s surface to the top of the atmosphere. Areas with values less than 220 Dobson Units are considered to have experienced severe ozone destruction. The image below presents a color-coded scale for Dobson Units that shows the 220-unit cut-off below which the concentration of ozone is recognized as being low enough that it poses a danger to human health. Please note which colors represent high ozone concentrations and which colors represent low ozone concentrations.
6.2 U3 Ultraviolet radiation reaching the surface of the Earth damages human living tissues, increasing the incidence of cataracts, mutation during cell division, skin cancer and other subsequent effects on health.
- Explain the effects of depleting stratospheric ozone.
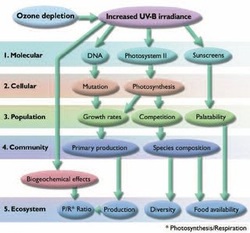 image from www.eoearth.org
image from www.eoearth.org
Effects of UV on living organisms
- Increase in mutation rates in DNA causing cancer
- Can cause eye cataracts
- Can damage the ability to carry out photosynthesis in plants and phytoplankton
- Reduces primary production and therefore total productivity
- Damage to immune system
6.2.U4 The effects of increased ultraviolet radiation on biological productivity include damage to photosynthetic organisms, especially phytoplankton, which form the basis of aquatic food webs.
- Explain the effects of depleting stratospheric ozone.
UV-B radiation has been shown to be harmful to living organisms, damaging DNA, proteins, lipids and membranes. Plants, which use sunlight for photosynthesis and are unable to avoid exposure to enhanced levels of UV-B radiation, are at risk. UV-B impairs photosynthesis in many species. Overexposure to UV-B reduces size, productivity, and quality in many of the crop plant species that have been studied (among them, many varieties of rice, soybeans, winter wheat, cotton, and corn). Similarly, overexposure to UV-B impairs the productivity of phytoplankton in aquatic ecosystems. UV-B increases plant's’ susceptibility to disease. The effects also include mutation and subsequent effects on health and damage to photosynthetic organisms, especially phytoplankton and their consumers such as zooplankton.
6.2.U5 Pollution management may be achieved by reducing the manufacture and release of ozone-depleting substances. Methods for this reduction include:
- recycling refrigerants
- developing alternatives to gas-blown plastics, halogenated pesticides, propellants and aerosols
- developing non-propellant alternatives.
- Discuss the approaches taken to reduce stratospheric ozone depletion.

Altering Human Activity
NOTE: There is no real application for the third phase of the pollution management model as recovery is reliant on time after the removal of ODSs
- Replace gas-blown plastics
- Replace the old fridge with the existing "greenfreeze" technology which does not deplete the ozone
- Propane and butane have replaced CFCs as a propellant.
- Non-propellant alternatives
- Methyl bromide can be replaced with other pesticides
- Recover and recycle CFCs
- Capture CFCs from scrap car AC units
- Ban on production and use of ODS
- National legislation
- Policing and enforcement to ensure compliance and stop illegal use of ODS.
- Add ozone to or remove chlorine from stratosphere
- Removal and destruction of existing ODS in refrigerators and air conditioning systems.
NOTE: There is no real application for the third phase of the pollution management model as recovery is reliant on time after the removal of ODSs
6.2.U6 UNEP has had a key role in providing information, and creating and evaluating international agreements, for the protection of stratospheric ozone.
- Discuss the role of the UN in protecting stratospheric ozone.

United Nations Environment Programme (UNEP) in forging international agreements
- ITIHC (International Trade in Harmful Chemicals)
- air pollution
- contamination of international waterways
- provide information to countries and public on disadvantages of pollution
- brought together 24 countries in 1987 to sign the initial Montreal Protocol on Substances that Deplete the Ozone Layer
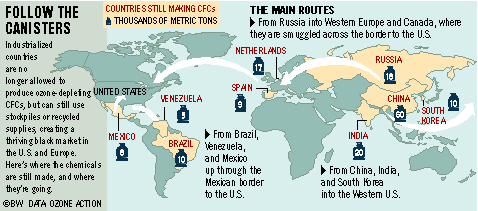
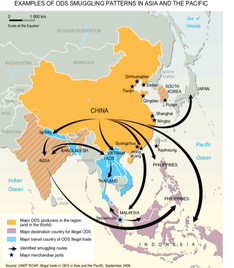
6.2.U7 An illegal market for ozone-depleting substances persists and requires consistent monitoring.
- Identify issues that still persist with ozone-depleting substances
 https://www.researchgate.net/figure/The-illegal-trade-in-ozone-depleting-substances-in-the-Asia-Pacific_fig9_304215688
https://www.researchgate.net/figure/The-illegal-trade-in-ozone-depleting-substances-in-the-Asia-Pacific_fig9_304215688
In many low-income countries, there is still a significant demand for CFCs as reliance on equipment using these chemicals remains high. The problem is made worse by the imports of used refrigeration and air-conditioning equipment.
The black market in ozone-depleting substances (ODS) is a direct consequence of international agreement on targets to reduce and phase-out the production and consumption of such chemicals. The illegal trade in CFCs is undermining the ‘Montreal Protocol on Substances that Deplete the Ozone Layer’.
Reasons for the illegal trade:
As a result of the decline in the production and use of CFCs, and the continuation of CFC production in developing countries, the lure of illegal trade in CFCs is obvious. Significant volumes of illegal imports of CFCs into Western Europe have been reported, even though production in Western Europe ceased at the end of 1994. Unfortunately, the Montreal Protocol currently does not require countries to implement controls against illegal trade, although they have been urged to install verification programs to reduce illegal trade in ODCs.
The black market in ozone-depleting substances (ODS) is a direct consequence of international agreement on targets to reduce and phase-out the production and consumption of such chemicals. The illegal trade in CFCs is undermining the ‘Montreal Protocol on Substances that Deplete the Ozone Layer’.
Reasons for the illegal trade:
- ODS substitutes are often costlier than CFCs
- Updating equipment to enable use of alternative chemicals is expensive
- The lifetime of CFC containing equipment is long
- Penalties in may countries are small.
As a result of the decline in the production and use of CFCs, and the continuation of CFC production in developing countries, the lure of illegal trade in CFCs is obvious. Significant volumes of illegal imports of CFCs into Western Europe have been reported, even though production in Western Europe ceased at the end of 1994. Unfortunately, the Montreal Protocol currently does not require countries to implement controls against illegal trade, although they have been urged to install verification programs to reduce illegal trade in ODCs.
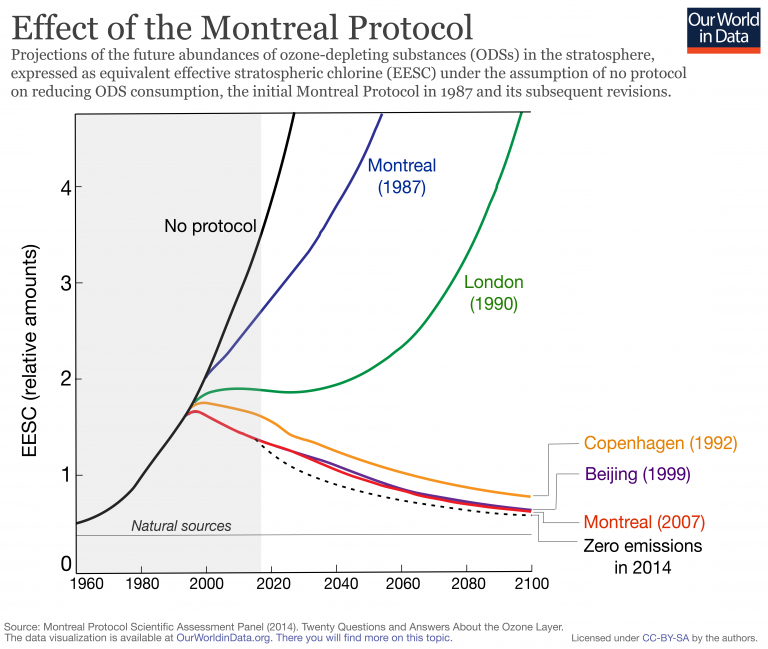
6.2.U8 The Montreal Protocol on Substances that Deplete the Ozone Layer (1987) and subsequent updates is an international agreement for the reduction of use of ozone-depleting substances signed under the direction of UNEP. National governments complying with the agreement made national laws and regulations to decrease the consumption and production of halogenated organic gases such as chlorofluorocarbons (CFCs).
1997 Montreal Protocol
Evaluate the use of ozone-depleting substances, and study the relative effectiveness of these agreements and the difficulties in implementing and enforcing them. Be familiar with what steps national governments are taking to comply with these agreements.
- international agreement on the emission of ozone-depleting substances
- froze production and consumption of CFC’s with goal of zero production by year 2000
- LEDC’s granted a longer time to implement the treaty
- China and India have not met their quotas under the MP because of their rapid economic growth and high demand for refrigeration & AC’s
- good example of a successful international cooperative effort to alter human impact on the environment
Evaluate the use of ozone-depleting substances, and study the relative effectiveness of these agreements and the difficulties in implementing and enforcing them. Be familiar with what steps national governments are taking to comply with these agreements.
Applications and skills:
6.2.A1 Evaluate the role of national and international organizations in reducing the emissions of ozone-depleting substances.
- Evaluate the effectiveness of the Montreal Protocol.
UNEP (United Nations Environment Programme), forges international agreements, studies the effectiveness of these agreements, and the difficulties implementing and enforcing them.
- The Montreal Protocol (1987), which is an international agreement on reduction of emission of ozone-depleting substances.
- The signatories agreed to freeze production of many CFCs and halons and strongly reduce consumption and production of these substances by 2000.
- People from many different backgrounds have come together from across the world including scientists, industrialists, researchers, policy and law makers, to work together on a common cause.
- Most countries followed the rules but China and India continued to produce and use huge amounts of CFCs.
- But they have since both agreed to phase out the use of CFCs.
- Therefore, the Montreal Protocol is often used as an exemplar of successful international cooperation
Key Terms
|
zooplankton
phytoplankton stratosphere layered structure polar thinning |
gas-blown plastics
chlorofluorocarbon composition positive feedback photodissociation |
ozone-depleting substances (ODS)
halogenated organic gases ultraviolet (UV) radiation non-point source |
UNEP
ozone lapse rate freon halon |
Montreal Protocol
methyl bromide chlorine non-point source |
Classroom Materials
Monitoring Tropospheric Ozone Lab
The Ozone Layer Lab
Montreal Protocol Case Study
Montreal Protocol article
Ozone depletion vs Global Warming
Our World Data Ozone Database
Case Study
Ozone Case Study
Monitoring Tropospheric Ozone Lab
The Ozone Layer Lab
Montreal Protocol Case Study
Montreal Protocol article
Ozone depletion vs Global Warming
Our World Data Ozone Database
Case Study
- One detailed case study evaluating the role of national and international organizations in reducing the emissions of ozone-depleting substances (eg. the Montreal Protocol)
Ozone Case Study
Powerpoint and Notes Adapted from Brad Kremer and Masfar
Your browser does not support viewing this document. Click here to download the document.
Your browser does not support viewing this document. Click here to download the document.
Correct use of terminology is a key skill in ESS. It is essential to use key terms correctly when communicating your understanding, particularly in assessments. Use the quizlet flashcards or other tools such as learn, scatter, space race, speller and test to help you master the vocabulary.
Useful Links
Ozone formation and destruction, as well as its link to global warming - Dynamic Science
Ozone - EPA
NASA’s Earth Observatory page on tropospheric ozone - NASA
Webster’s Online Dictionary entry for ozone
Tropospheric ozone processes. - Belgium Institute of Space Aeronomy
Health Effect of UV radiation - WHO
Positive and Negative Effects of UV Radiation - Science Learn
Convention on Long-Range Transboundary Air Pollution (LRTAP), Geneva, 1979 - Wikipedia
Vienna Convention for the Protection of the Ozone Layer, Vienna, 1985 - Wikipedia
Montreal Protocol on Substances that Deplete the Ozone Layer, Montreal 1987 - Wikipedia
In The News
Nations, Fighting Powerful Refrigerant That Warms Planet, Reach Landmark Deal - New York Times October 2016
Greenhouse gases deal will make little difference to west - Guardian Oct 2016
Ozone depletion: SA to phase out harmful gases but struggles to find suitable alternatives - Traveller24 Oct 2016
Ozone Hole Shows Signs of Shrinking, Scientists Say - New York Times Jun 2016
Ultraviolet Radiation: How It Affects Life on Earth - NASA · September 6, 2001
Nations, Fighting Powerful Refrigerant That Warms Planet, Reach Landmark Deal - New York Times October 2016
Greenhouse gases deal will make little difference to west - Guardian Oct 2016
Ozone depletion: SA to phase out harmful gases but struggles to find suitable alternatives - Traveller24 Oct 2016
Ozone Hole Shows Signs of Shrinking, Scientists Say - New York Times Jun 2016
Ultraviolet Radiation: How It Affects Life on Earth - NASA · September 6, 2001
International-mindedness:
- The depletion of ozone has global implications to ocean productivity and oxygen production.
- National economic approaches may have an impact on international environmental discussions.
TOK
- The Montreal Protocol was an international agreement created by the UN—can one group or organization decide what is best for the rest of the world?
Video Clips
CFCs and Ozone
NASA's been tracking the thinning of the ozone layer the south pole for nearly 20 years. In this slowed-down animation, you can watch the ozone hole's average size each October.
Restrictions on chemicals credited in ozone-hole shrinkage
The signing of the Montreal Protocol in September 1987 launched an unprecedented global effort in the protection of the environment. To this day, the Vienna Convention and the Montreal Protocol are the only universally ratified treaties, uniting 198 countries in taking on the fight against man-made ozone depleting substances.
Nearly 200 countries have agreed on a global deal to reduce greenhouse gases mostly used in refrigerators and air conditioners.