topic 2.6: structure of dna and rna
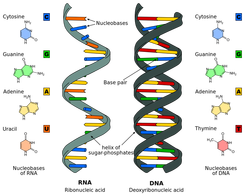 image from http://www.differencebetween.net/
image from http://www.differencebetween.net/
In the DNA Structure unit students are learn to the structure of DNA. The structure of DNA molecule is vital for understanding the replication and functioning of the molecule.
The unit is planned to take 2 school days.
The unit is planned to take 2 school days.
Essential idea:
- The structure of DNA allows efficient storage of genetic information.
Nature of science:
- Using models as representation of the real world—Crick and Watson used model making to discover the structure of DNA. (1.10)
- List types of models used in science.
- State a common feature of models in science.
- List ways in which models are different from the structure or process it represents.
- List types of models used in science.
Understanding
2.6.U1 The nucleic acids DNA and RNA are polymers of nucleotides. (Oxford Biology Course Companion page 106).
- State the two types of nucleic acid.
- State the two types of nucleic acid.
- Outline the parts of a nucleotide.
- Identify and label carbons by number (for example, C1, C2, C3) on a nucleotide drawing.
- Explain how nucleotides can connect to form a nucleic acid polymer.
- State the names of the nitrogenous bases found in DNA and RNA.
- Identify nitrogenous bases as either a pyrimidine or purine.
- State the complementary base pairing rules.
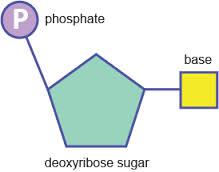
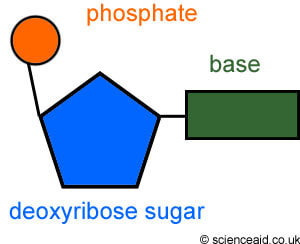
Nucleotides consist of three parts:
Both the phosphate group and nitrogenous base are attached to the central pentose sugar
The nitrogenous base is attached to the 1’– carbon atom (right point)
The phosphate base is attached to the 5’– carbon atom (left point)
- Sugar has five carbon atoms meaning it a pentose sugar
- a phosphate group, acidic, negatively-charged part of nucleic acids
- base that contains nitrogen and has either one or two rings of atoms in it’s structure
Both the phosphate group and nitrogenous base are attached to the central pentose sugar
The nitrogenous base is attached to the 1’– carbon atom (right point)
The phosphate base is attached to the 5’– carbon atom (left point)
The base and phosphate are both linked together by covalent bonds to the pentose sugar.
The four different nucleotides can be linked together in any sequence because the phosphate and sugar used to link them are the same in every nucleotide. Any base sequence is possinle along a DNA or RNA molecule.
This is the key to nucleic acids acting as a store of genetic information. Base sequence = store of information. Sugar phosphate backbone = ensures that the store is stable and secure
- covalent bonds are formed between the phosphate of one nucleotide and the pentose sugar of the next nucleotide, creating a strong backbone for the molecule of alternating sugar and phosphate groups, with a base linked to each sugar
- different bases in both DNA & RNA = Four different nucleotides
The four different nucleotides can be linked together in any sequence because the phosphate and sugar used to link them are the same in every nucleotide. Any base sequence is possinle along a DNA or RNA molecule.
This is the key to nucleic acids acting as a store of genetic information. Base sequence = store of information. Sugar phosphate backbone = ensures that the store is stable and secure
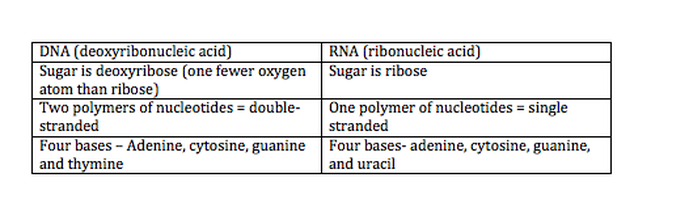
2.6.U2 DNA differs from RNA in the number of strands present, the base composition and the type of pentose. (Oxford Biology Course Companion page 106).
- Compare the structure of DNA and RNA.
There are two types of nucleic acids present in cells – DNA and RNA
- DNA (deoxyribonucleic acid) is a more stable double stranded form that stores the genetic blueprint for cells
- RNA (ribonucleic acid) is a more versatile single stranded form that transfers the genetic information for decoding
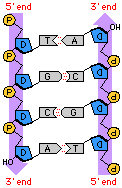
2.6.U3 DNA is a double helix made of two antiparallel strands of nucleotides linked by hydrogen bonding between complementary base pairs. (Oxford Biology Course Companion page 108).
- Define antiparallel in relation to DNA structure.
- Outline the formation of a DNA double helix by hydrogen bonding between nitrogenous bases.
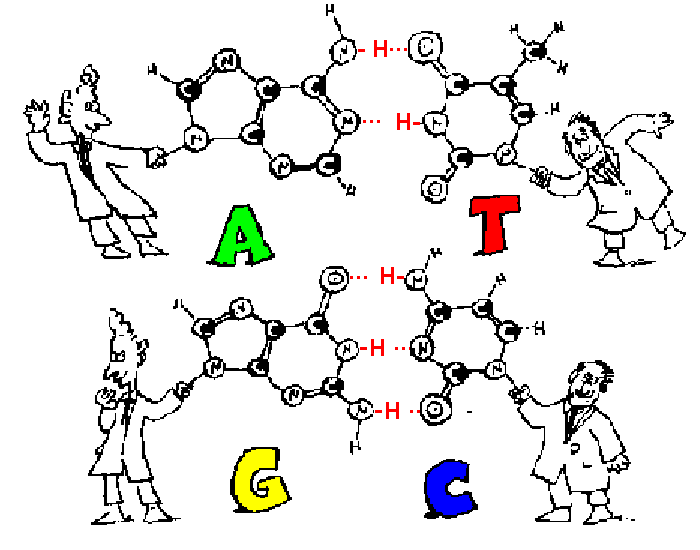
- Identify the four bases of DNA based on the numbers of rings (purines or pyrimidines) and the number of hydrogen bonds it can form.
- State the number of nitrogenous bases per complete turn of the DNA double helix.
Nucleic acids are composed of nucleotide monomers which are linked into a single strand via condensation reactions
Each strand has a chain of nucleotides linked by covalent bonds, two strands are parallel but run in opposite directions so they are antiparallel. As the antiparallel chains lengthen, the atoms will organise themselves into the most stable energy configuration. This atomic arrangement results in the double-stranded DNA forming a double helix (~10 – 15 bases per twist)
The strands are held together by hydrogen bonds between the nitrogenous bases
This is called complementary base pairing – A and T complement each other by forming base pairs and same with G and C
- The phosphate group of one nucleotide attaches to the sugar of another nucleotide (at the 3’– hydroxyl (-OH) group)
- This results in a phosphodiester bond forming between the two nucleotides (and water is produced as a by-product)
- Successive condensation reactions result in the formation of long polynucleotide strands
Each strand has a chain of nucleotides linked by covalent bonds, two strands are parallel but run in opposite directions so they are antiparallel. As the antiparallel chains lengthen, the atoms will organise themselves into the most stable energy configuration. This atomic arrangement results in the double-stranded DNA forming a double helix (~10 – 15 bases per twist)
The strands are held together by hydrogen bonds between the nitrogenous bases
- Adenine (A) & thymine (T)
- Guanine (G) & cytosine (C)
This is called complementary base pairing – A and T complement each other by forming base pairs and same with G and C
Application:
2.6.A1 Crick and Watson’s elucidation of the structure of DNA using model making. (Oxford Biology Course Companion page 110).
- Outline the role of Chargaff, Watson, Crick, Franklin and Wilkins in the discovery of DNA structure.
- Explain how Watson and Crick used model building to determine the structure of DNA.
The structural organisation of the DNA molecule was correctly proposed in 1953 by James Watson and Francis Crick. These scientists constructed models to quickly visualise and assess the viability of potential structures. Their efforts were guided by an understanding of molecular distances and bond angles developed by Linus Pauling, and were based upon some key experimental discoveries:
Using trial and error, Watson and Crick were able to assemble a DNA model that demonstrated the following:
As Watson and Crick’s model building was based on trial and error, a number of early models possessed faults:
The Rosalind Franklin Controversy
The final construction of a correct DNA molecule owed heavily to the X-ray crystallography data generated by Rosalind Franklin. This data confirmed the arrangement of the DNA strands into a helical structure. The data was shared without Franklin’s knowledge or permission and contributed profoundly to the final design. Hence, Franklin is now recognised as a key contributor to the elucidation of DNA structure
- DNA is composed of nucleotides made up of a sugar, phosphate and base – Phoebus Levene, 1919
- DNA is composed of an equal number of purines (A + G) and pyrimidines (C + T) – Erwin Chargaff, 1950
- DNA is organised into a helical structure – Rosalind Franklin, 1953 (data shared without permission)
Using trial and error, Watson and Crick were able to assemble a DNA model that demonstrated the following:
- DNA strands are antiparallel and form a double helix
- DNA strands pair via complementary base pairing (A = T ; C Ξ G)
- Outer edges of bases remain exposed (allows access to replicative and transcriptional proteins)
As Watson and Crick’s model building was based on trial and error, a number of early models possessed faults:
- The first model generated was a triple helix
- Early models had bases on the outside and sugar-phosphate residues in the centre
- Nitrogenous bases were not initially configured correctly and hence did not demonstrate complementarity
The Rosalind Franklin Controversy
The final construction of a correct DNA molecule owed heavily to the X-ray crystallography data generated by Rosalind Franklin. This data confirmed the arrangement of the DNA strands into a helical structure. The data was shared without Franklin’s knowledge or permission and contributed profoundly to the final design. Hence, Franklin is now recognised as a key contributor to the elucidation of DNA structure
Skill
2.6.S1 Drawing simple diagrams of the structure of single nucleotides of DNA and RNA, using circles, pentagons and rectangles to represent phosphates, pentoses and bases. (Oxford Biology Course Companion page 107).
- Draw the basic structure of a single nucleotide (using circle, pentagon and rectangle).
- Draw a simple diagram of the structure of RNA.
- Draw a simple diagram of the structure of DNA.
- Identify and label the 5’ and 3’ ends on a DNA or RNA diagram
Both the phosphate group and nitrogenous base are attached to the central pentose sugar
The nitrogenous base is attached to the 1’– carbon atom (right point)
The phosphate base is attached to the 5’– carbon atom (left point)Circles for phosphate
The nitrogenous base is attached to the 1’– carbon atom (right point)
The phosphate base is attached to the 5’– carbon atom (left point)Circles for phosphate
- pentagons for pentose sugar
- rectangles for bases
- C, carbon atom is on the right hand side of the pentose sugar
- phosphate is linked to C5, the carbon atom on the side chain on the upper left side of the pentose sugar
Key Terms:
|
3'
micropipette 5' cytosine pentose deoxyribonucleic acid X ray diffraction base pair ruling |
adenine
nitrogenous base phosphate deoxyribose polymer Watson ribose |
antiparrallel
nucleic acid double helix nucleoid purine pyrimidine base |
chromatin
nucleotides nucleosome chromosome nucleotide guanine site |
nucleus
Crick thymine histone hydrogen bond Franklin Chargaff |
Class Materials:
DNA Model
DNA Replication Worksheet
Nucleic acids web assignment
The Race for the Double Helix video
DNA History Readings
Envisioning DNA
Watson and Crick paper
Rosalind Franklin's Story
DNA components worksheet
DNA model assignment
Modeling an actual DNA sequence
Play-doh models
Topic 2.6 Review
DNA Model
DNA Replication Worksheet
Nucleic acids web assignment
The Race for the Double Helix video
DNA History Readings
Envisioning DNA
Watson and Crick paper
Rosalind Franklin's Story
DNA components worksheet
DNA model assignment
Modeling an actual DNA sequence
Play-doh models
Topic 2.6 Review
Powerpoint and Notes on Topic 2.6 by Chris Payne
Your browser does not support viewing this document. Click here to download the document.
Your browser does not support viewing this document. Click here to download the document.
Correct use of terminology is a key skill in Biology. It is essential to use key terms correctly when communicating your understanding, particularly in assessments. Use the quizlet flashcards or other tools such as learn, scatter, space race, speller and test to help you master the vocabulary.
Helpful Links:
A decent tour of the basics, from Learn.Genetics.
Journey Into DNA
DNA anatomy
DNA Coiling To Form Chromosomes
Chromosome Description
Click here to access Pearson Education. Inset the express code 4273P and click on Weblink 3.5
In The News
Counting All the DNA on Earth - New York Times, July 2015
DNA's dynamic nature makes it well-suited to serve as the blueprint of life - Science Daily, August 2016
TOK:
- The story of the elucidation of the structure of DNA illustrates that cooperation and collaboration among scientists exists alongside competition between research groups. To what extent is research in secret ‘anti-scientific’?
- What is the relationship between shared and personal knowledge in the natural sciences?
Video Clips:
The Race for the DNA Model
The discovery of the structure of the DNA double helix was one of the most important of the 20th century. In this educational video, explore Watson and Crick’s quest to understand DNA’s structure, and Rosalind Franklin’s key insights via x-ray crystallography.
The discovery of the structure of DNA was one of the most important scientific achievements in human history. The now-famous double helix is almost synonymous with Watson and Crick, two of the scientists who won the Nobel prize for figuring it out. But there’s another name you may not know:
An exploration of the structure of deoxyribonucleic acid, or DNA.
Biology textbooks are full of drawings of DNA, but none of those show what DNA actually looks like. Sure, they’re good models for understanding how DNA works, but inside of real cells, it’s a whole lot more interesting. Learn why we can’t look directly at DNA, and find out how DNA is actually packed inside cells.
Hank introduces us to that wondrous molecule deoxyribonucleic acid - also known as DNA - and explains how it replicates itself in our cells.
Paul Andersen describes the molecular structure of DNA. He describes the major parts of a nucleotide and explains how they are assembled into a nucleic acid. The nitrogenous base, deoxyribose sugar and phosphate group make up a single nucleotide. The 5' and 3' end of DNA is described. The importance of hydrogen bonds in the 3-dimensional shape is also included