Topic 2.3: Carbohydrates and lipids
In the Basics of Biochemistry unit students are introduced to the major classes of biologically important molecules and the types of reactions used to build and break apart those molecules. The structure and function of water, as the medium of life, is also a focus.
The unit is planned to take 2 school days.
The unit is planned to take 2 school days.
Essential idea:
- Compounds of carbon, hydrogen and oxygen are used to supply and store energy.
Nature of Science
- Evaluating claims—health claims made about lipids in diets need to be assessed. (5.2)
- Describe how the effect of lipids on health can be assessed scientifically.
- Describe how the effect of lipids on health can be assessed scientifically.
Understandings:
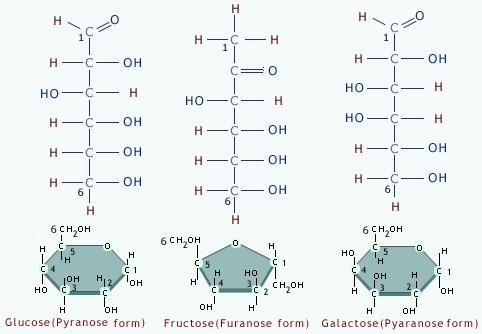
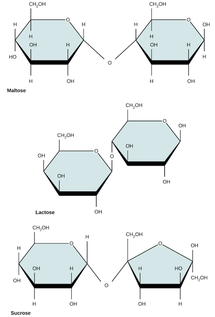
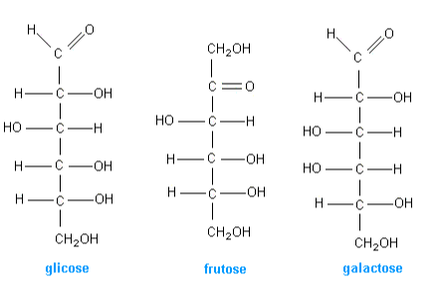
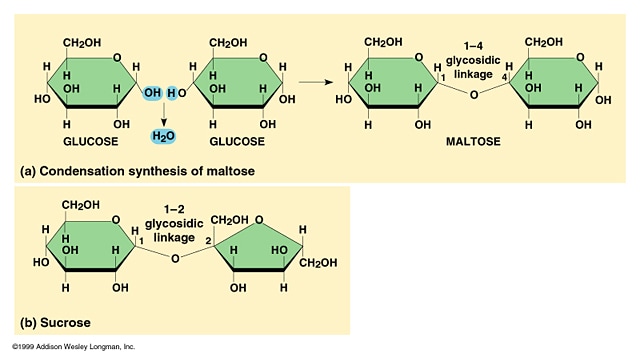
2.3.U.1 Monosaccharide monomers are linked together by condensation reactions to form disaccharides and polysaccharide polymers.(Oxford Biology Course Companion page 74).
- Define monosaccharide, disaccharide and polysaccharide.
- List three examples of monosaccharides.
- List three examples of disaccharides.
- List three examples of polysaccharides.
- Use molecular diagrams to draw the formation of maltose from two glucose monomers.
- Explain a condensation reaction connecting two monosaccharides in the formation of a disaccharide.
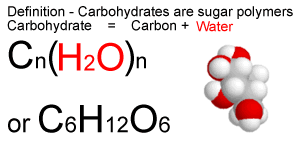
Carbohydrates (also called saccharides) are molecular compounds made from just three elements: carbon, hydrogen and oxygen. Monosaccharides (e.g. glucose) and disaccharides (e.g. sucrose) are relatively small molecules. They are often called sugars. Other carbohydrate molecules are very large (polysaccharides such as starch and cellulose).
Carbohydrates are:
Carbohydrates are:
- a source of energy for the body e.g. glucose and a store of energy, e.g. starch in plants
- building blocks for polysaccharides (giant carbohydrates), e.g. cellulose in plants and glycogen in the human body
- components of other molecules eg DNA, RNA, glycolipids, glycoproteins, ATP
|
monosaccharides:
|
disaccharides
|
polysaccharides
|
Monosaccharides:
Disaccharides
Polysaccharides
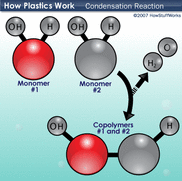
Condensation reactions make bonds.
Hydrolysis bonds break these bonds.
- condensation synthesis reactions link two monosaccharide monomers
- forming one disaccharide molecule and one H2O molecule
- repeated additions of monosaccharides produces a polysaccharide
Hydrolysis bonds break these bonds.
- a polysaccharides can be broken down into monosaccharides
- H2O molecules used as a sources of -H and a -OH groups
- catalyzed by enzymes
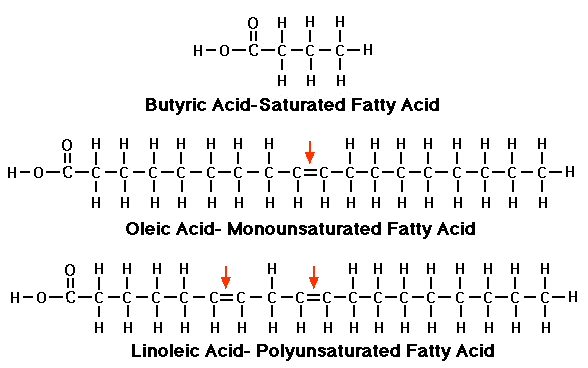
2.3.U.2 Fatty acids can be saturated, monounsaturated or polyunsaturated. (Oxford Biology Course Companion page 81).
- Describe the differences between saturated and unsaturated (mono- or poly-) fatty acids.
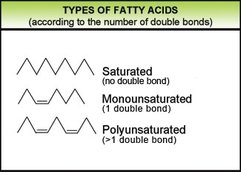
Fats are a group of chemical compounds that contain fatty acids. Energy is stored in the body mostly in the form of fat. Fat is also needed in the diet to supply essential fatty acids that are substances essential for growth but not produced by the body itself
There are three main types of fatty acids: saturated, monounsaturated and polyunsaturated. All fatty acids are chains of carbon atoms with hydrogen atoms attached to the carbon atoms.
Saturated fatty acid
Monounsaturated fatty acid
Polyunsaturated fattyacid.
There are three main types of fatty acids: saturated, monounsaturated and polyunsaturated. All fatty acids are chains of carbon atoms with hydrogen atoms attached to the carbon atoms.
Saturated fatty acid
- Hydrogen atoms attached to every carbon atom.
- All of the carbons are attached to each other with single bonds.
Monounsaturated fatty acid
- A pair of hydrogen atoms in the middle of a chain is missing, creating a single gap that leaves two carbon atoms connected by a double bond rather than a single bond.
Polyunsaturated fattyacid.
- A pair of hydrogen atoms in the middle of a chain is missing, having more than one gap that leaves two carbon atoms connected by a double bond rather than a single bond."
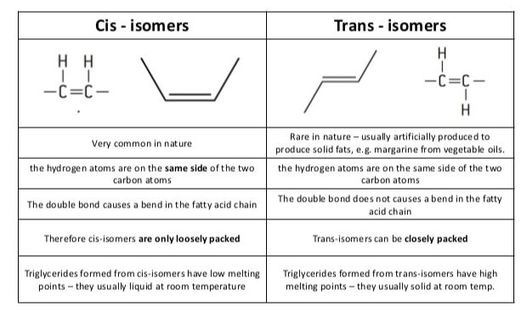
2.3.U.3 Unsaturated fatty acids can be cis or trans isomers. (Oxford Biology Course Companion page 82).Describe the differences between cis- and trans- fatty acids.
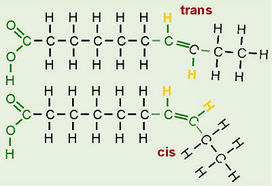
While a saturated fatty acid is a straight molecule on the average, the double bond in an unsaturated fatty acid produces a kink in the molecule. This is because a double bond cannot rotate. The bend in the carbon chain is much more pronounced in the cis isomer compared to the trans isomer. For this reason, cis fatty acids (and triacylglycerols made from them) do not solidify as readily as trans fatty acids. Due to the larger bend, the cis isomers cannot line up next to one another in as ordered a fashion as the trans isomers.
The configuration of the double bond in an unsaturated fatty acid can take two forms (or, to chemists, isomers): the naturally predominant cis form, in which both of the hydrogen atoms are on the same side of the chain; and the uncommon-in-nature trans isomer, in which the hydrogen atoms are on opposite sides. The trans form is (in most cases) best thought of as 'damaged'.
The configuration of the double bond in an unsaturated fatty acid can take two forms (or, to chemists, isomers): the naturally predominant cis form, in which both of the hydrogen atoms are on the same side of the chain; and the uncommon-in-nature trans isomer, in which the hydrogen atoms are on opposite sides. The trans form is (in most cases) best thought of as 'damaged'.
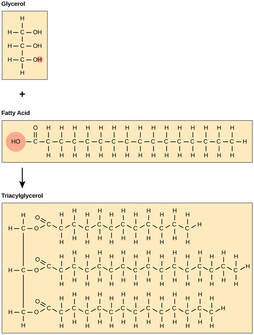
2.3.U.4 Triglycerides are formed by condensation from three fatty acids and one glycerol. (Oxford Biology Course Companion page 77).
- Outline the difference between fats and oils.
- Explain a condensation reaction connecting fatty acids and glycerol to form a triglyceride.
- State two functions of triglycerides.
Triglycerides are lipids consisting of one glycerol molecule bonded with three fatty acid molecules. The bonds between the molecules are covalent and are called Ester bonds. They are formed during a condensation reaction. Triglycerides are hydrophobic and so insoluble in water. The charges are evenly distributed around the molecule so hydrogen bonds to not form with water molecules.
Applications
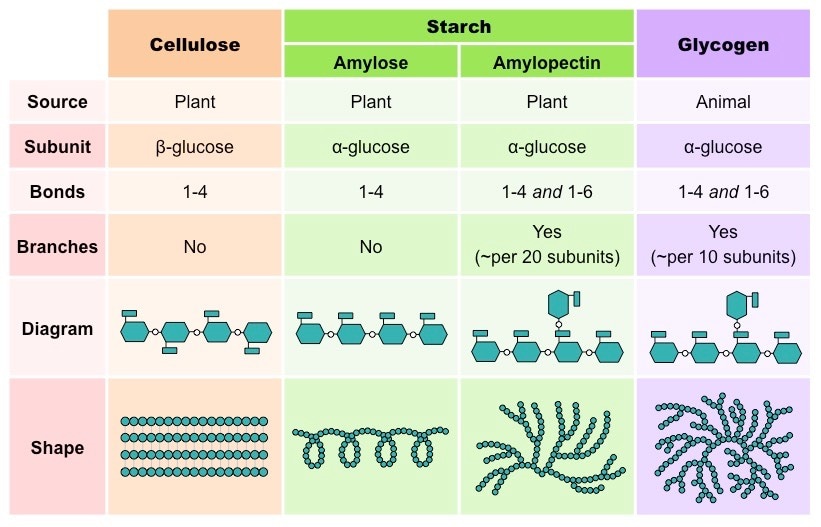
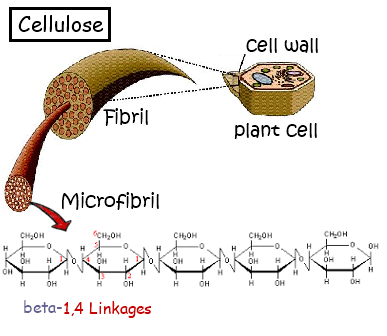
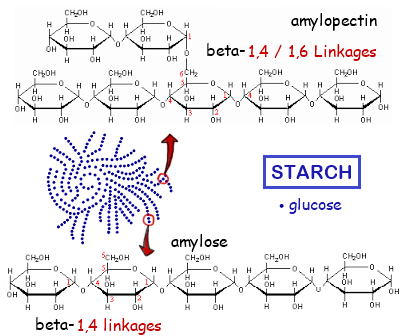
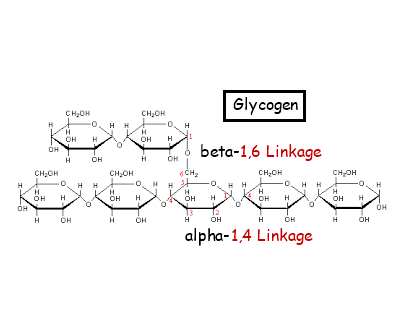
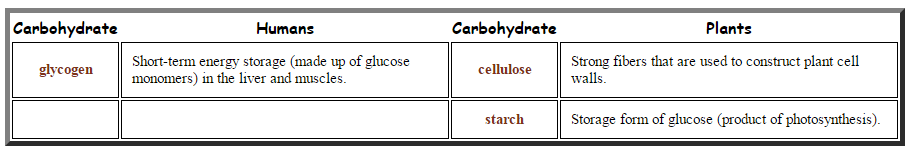
2.3.A.1 Structure and function of cellulose and starch in plants and glycogen in humans. (Oxford Biology Course Companion page 76).
- State the structural difference between alpha and beta glucose.
- Contrast the structure and functions of cellulose, amylose, amylopectin and glycogen.
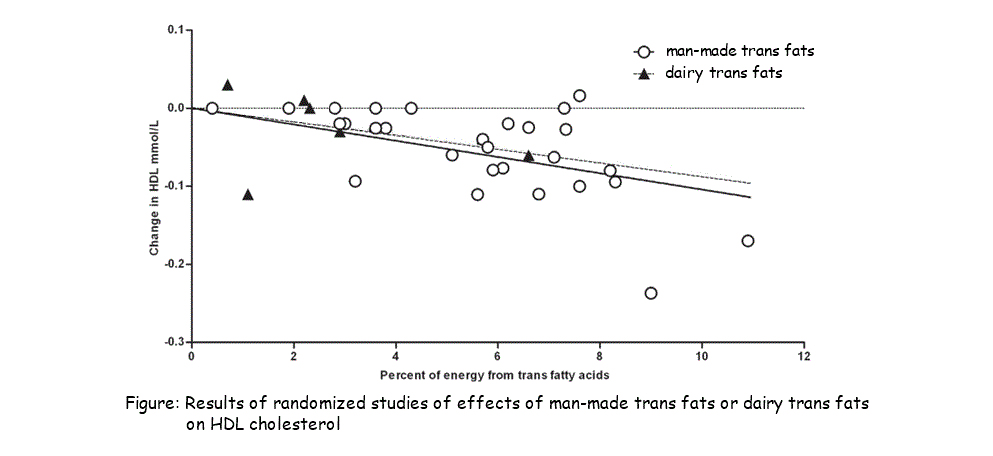
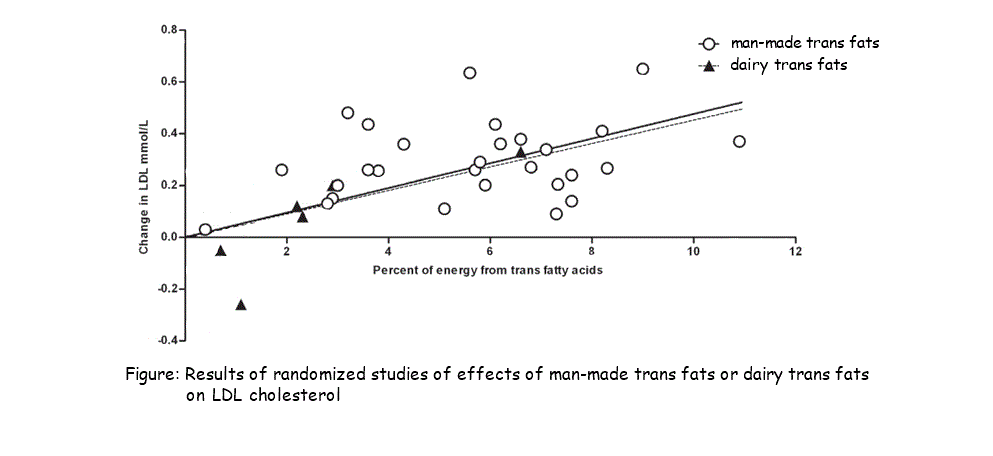
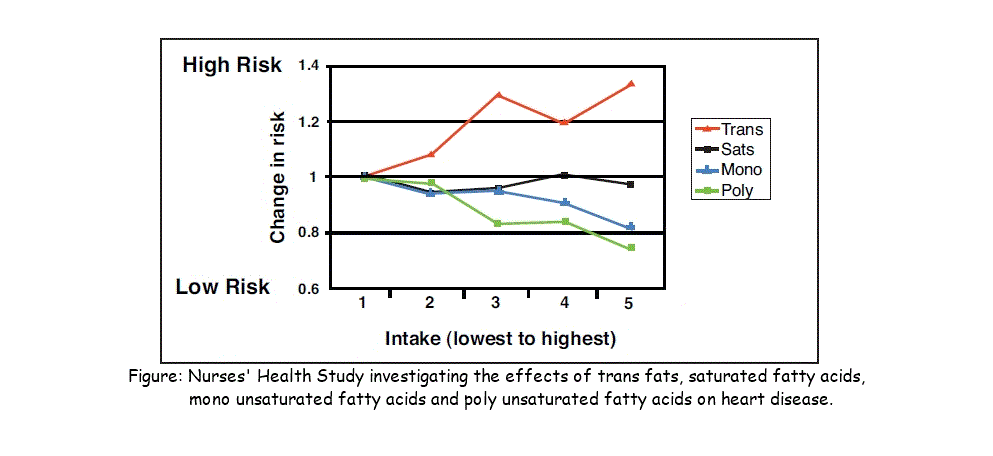
2.3.A.2 Scientific evidence for health risks of trans fats and saturated fatty acids. (Oxford Biology Course Companion page 83).
- Discuss the relationship between saturated fatty acids and/or trans fat intake and rates of coronary heart disease.
Many factors affect heart disease, however, diets low in saturated fat and cholesterol may reduce the risk of this disease. Cholesterol carried throughout the body as low density lipoproteins (LDL) and high density lipoproteins (HDL).. Cholesterol in LDSs get deposited in the walls of blook vessels whild HDLs revome cholesterol from blood vessels. Research show that high LDL, low HDL levels in blood increase risk of heart disease and low LDL, high HDL levels reduce risk of heart disease. Therefore, important for diet to reduce LDL levels and increase HDL levels
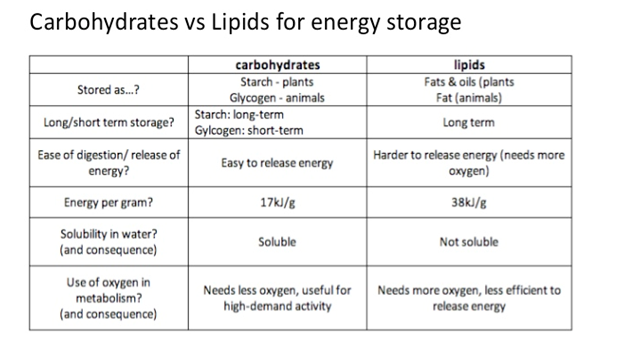
2.3.A.3 Lipids are more suitable for long-term energy storage in humans than carbohydrates. (Oxford Biology Course Companion page 78).
- Explain the energy storage of lipids compared to that of carbohydrates.
Carbohydrates and lipids can both be used as energy storage however carbohydrates are usually used for short term storage whereas lipids are used for long term storage. Carbohydrates are soluble in water unlike lipids. This makes carbohydrates easy to transport around the body (from and to the store). Also, carbohydrates are a lot easier and more rapidly digested so their energy is useful if the body requires energy fast. As for lipids, they are insoluble which makes them more difficult to transport however because they are insoluble, lipids do not have an effect on osmosis which prevents problems within the cells in the body. They also contain more energy per gram than carbohydrates which makes lipids a lighter store compared to a store of carbohydrates equivalent in energy
2.3.A.4 Evaluation of evidence and the methods used to obtain the evidence for health claims made about lipids. (Oxford Biology Course Companion page 85).
- Define evaluation in respect to evidence from and methods of research.
- Outline the manner in which the implications of research can be assessed.
- Outline the manner in which the limitations of research can be assessed.
- Evaluate a given health claim made about lipids.
Most research looking at the health claims of diets or nutritional components in a diet are based on observational studies. Outlined below are the differences between observational studies and experimental tests and considerations that need to be taken when making any conclusions regarding observational studies
Observational Studies:
Experimental Tests
Observational Studies:
- these studies only give you correlations and do not allow for establishing the causal relationship between the phenomena studied,
- the research observes what happens to people under exposure conditions that have been self-selected or have been determined by influences outside the control of the researcher
- these studies, regardless of their size or number, only provide hypothesis generating data.regardless of their size or number, only provide hypothesis given have to be rigorously generating data
Experimental Tests
- uses hypothesis then have to be rigorously tested using the scientific process
- the research can choose what conditions to study by controlling all key factors except those being test
- the researcher observes what happens to people under exposure conditions that have been self-selected or have been determined by influences outside the control of the research
Skills:
2.3.S.1 Use of molecular visualization software to compare cellulose, starch and glycogen. (Oxford Biology Course Companion page 75).
- Demonstrate use of JMol to view molecular structures, including changing image size, rotating the image and changing the style of the molecular model.
- Identify carbon, hydrogen and oxygen atoms by color.
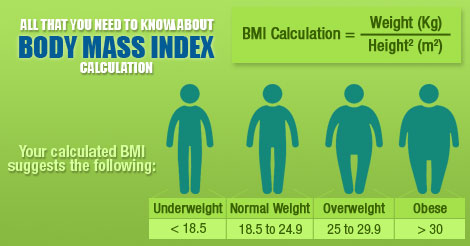
2.3.S.2 Determination body mass index by calculation or use of a nomogram. Oxford Biology Course Companion page 80).
- Calculate BMI using the formula.
- Determine BMI using a nomogram.
- Outline effects of a BMI that is too high or too low.
 Hover over the picture to access the BMI Calculator
Hover over the picture to access the BMI Calculator
Body mass index, or BMI, is used to determine whether you are in a healthy weight range for your height.
It is useful consider BMI alongside waist circumference, as increases or decreases in weight outside the healthy range may increase your health risks.
It is useful consider BMI alongside waist circumference, as increases or decreases in weight outside the healthy range may increase your health risks.

BMI compares your weight to your height, and is calculated by dividing your weight in kilograms by your height in metres squared.
It gives you an idea of whether you’re underweight, a healthy weight, overweight, or obese for your height. BMI is one type of tool to help health professionals to assess risk for chronic disease. Another important tool is waist circumference. It is also important to understand your other risk factors.
It gives you an idea of whether you’re underweight, a healthy weight, overweight, or obese for your height. BMI is one type of tool to help health professionals to assess risk for chronic disease. Another important tool is waist circumference. It is also important to understand your other risk factors.
Body Mass Index Nomogram
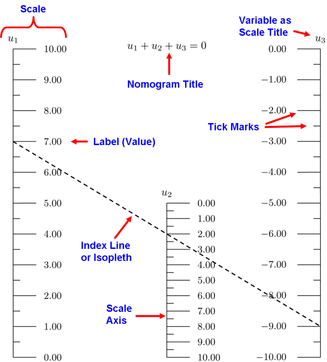
The Body Mass Index (BMI) Nomogram is a graph that shows a person's Body Mass Index as the point on the chart nearest the dashed line (representing the Body Mass Index) where height (in inches or centimetres) and weight (in pounds or kilograms) intersect.
Height is shown on the x-axis in centimetres or inches, and weight is shown on the y-axis in kilograms or pounds.
Dashed lines, representing the Body Mass Index, are displayed on the graph as calculated by the formula of weight (in kilograms) divided by height squared (in metres).
Height is shown on the x-axis in centimetres or inches, and weight is shown on the y-axis in kilograms or pounds.
Dashed lines, representing the Body Mass Index, are displayed on the graph as calculated by the formula of weight (in kilograms) divided by height squared (in metres).
Key Terms:
|
organic
carbohydrate monosaccharide disaccharide fatty acids cis triglycerides coronary heart disease |
inorganic
pentose hexose ribose lipids trans condensation energy storage |
fructose
isomer maltose sucrose polymer isomers fats BMI |
lactose
starch gylcogen cellulose monounsaturated polyunsaturated oils nomogram |
glucose
monomer polysaccharide chitin unsaturated glycerol amylose amylopectin |
Classroom Material:
Carbohydrates
Carbohydrates worksheet
Carbohydrate taste testing lab
Starch hydrolysis lab
Model of Life Kit Carbohydrate
Lipids
Lipids worksheet
Lipids worksheet answers
Molecule identification practice
Phat lab introduction (pdf)
Phat lab scoring sheet (pdf)
McMush, macromolecules in food lab (pdf)
Review of carbohydrates and lipid structure (pdf)
Models of Life Kit Lipids
Topic 2.3 Review Notes
Topic 2.3 Kahoot Review Quiz
Carbohydrates
Carbohydrates worksheet
Carbohydrate taste testing lab
Starch hydrolysis lab
Model of Life Kit Carbohydrate
Lipids
Lipids worksheet
Lipids worksheet answers
Molecule identification practice
Phat lab introduction (pdf)
Phat lab scoring sheet (pdf)
McMush, macromolecules in food lab (pdf)
Review of carbohydrates and lipid structure (pdf)
Models of Life Kit Lipids
Topic 2.3 Review Notes
Topic 2.3 Kahoot Review Quiz
Powerpoint and Notes on Topic 2.3 from Chris Payne
Your browser does not support viewing this document. Click here to download the document.
Your browser does not support viewing this document. Click here to download the document.
Correct use of terminology is a key skill in Biology. It is essential to use key terms correctly when communicating your understanding, particularly in assessments. Use the quizlet flashcards or other tools such as learn, scatter, space race, speller and test to help you master the vocabulary.
Useful Links:
The Macromolecules of Life
Carbohydrates
Biomolecules: The Carbohydrates
Sweet medicines reading
The true sweet science reading
Explanation and animation from National Louis University
Animated tutorial from WiscOnline
Condensation of carbohydrates animation
What happens when glucose is placed in water? from John Gianni
Lipids
Biomolecules: the lipids
Molecular structure of fat
Lipid Dehydration
Dehydration Synthesis and Hydrolysis
Four Types of Chemical Reactions
Here is a good animation on cholesterol.
Structures of Fats from HHMI
Lipids (and condensation animation) from National Louis University
Animated tutorial from WiscOnline
In The News:
Don't use body mass index to determine whether people are healthy - Science Daily, February 4, 2016
Does Putting On A Few Pounds Help You Cheat Death? - NPR, May 10, 2016
Higher BMI not associated with increased risk of heart attack or early death, twin study shows - Science Daily August 1, 2016
F.D.A. sets 2018 deadline to rid foods of trans fats - New York Times, June 16, 2015
The Food Industry's Influence In Nutrition Research - NPR, September 17, 2016
International-mindedness:
- Variation in the prevalence of different health problems around the world could be discussed including obesity, dietary energy deficiency, kwashiorkor, anorexia nervosa and coronary heart disease.
TOK
- There are conflicting views as to the harms and benefits of fats in diets. How do we decide between competing views?
Video Clips:
Paul Andersen begins by explaining the structure and purpose of carbohydrates. He describes and gives examples of monosaccharides, disaccharides, oligosaccharide and polysaccharides. He explains how they grow through dehydration reactions and shrink through hydrolysis
Paul Andersen describes the lipids (of the fats). He explains how they are an important source of energy but are also required to cell membranes. He explains how the hydrocarbon tails in triglycerides contain energy available for life. He also explains how phospholipids construct, and cholesterol molecules main the cell membrane.
As the narrative goes, fat is bad. Well, it's actually more nuanced than that. The type of fat you eat is more impactful on your health than the quantity. George Zaidan examines triglycerides, the varied molecules that make up fat, and how to identify which types of fat you are consuming.
The body mass index, better known as BMI, is a measure of obesity that has been in use for over 200 years. It was a formula created by Belgian mathematician Adolphe Quetelet. It takes your weight in kilograms divides and divides it by height in meters squared.
John Oliver discusses how and why media outlets so often report untrue or incomplete information as science.