Topic 2.4: proteins
In the Basics of Biochemistry unit students are introduced to the major classes of biologically important molecules and the types of reactions used to build and break apart those molecules. The structure and function of water, as the medium of life, is also a focus.
The unit is planned to take 3 school days.
The unit is planned to take 3 school days.
Essential idea:
- Proteins have a very wide range of functions in living organisms.
Nature of science:
- Looking for patterns, trends and discrepancies—most but not all organisms assemble proteins from the same amino acids. (3.1)
- Explain the trend of organisms assembly of polypeptides from the same amino acids.
- Describe a discrepancy of the trend of all organisms using the same amino acids to assemble polypeptides.
- Explain the trend of organisms assembly of polypeptides from the same amino acids.
Understandings:
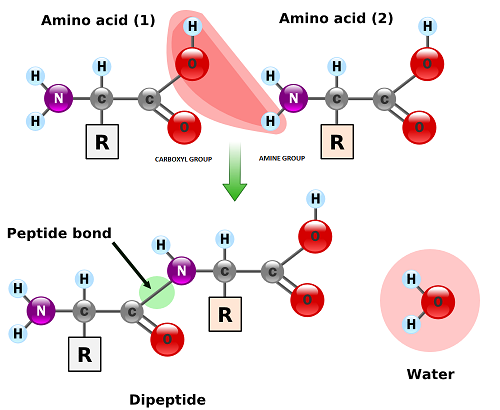
2.4.U.1 Amino acids are linked together by condensation to form polypeptides.
- Describe polypeptide chain formation in terms of the formation of peptide bonds and condensation reactions.
- Determine the number of peptide bonds given the number of amino acids in a polypeptide.
- Define dipeptide, oligopeptides and polypeptide.
Polypeptides are chains of amino acids that are made by linking together amino acids by condensation reactions- this happens on ribosomes by a process called translation. Amino acids are the main component of proteins and in many proteins they are the only component
Polypeptides can contain one protein some contain more
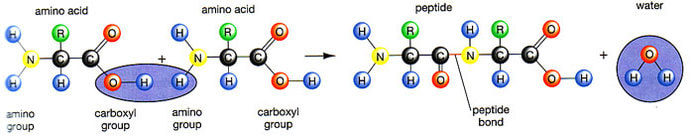
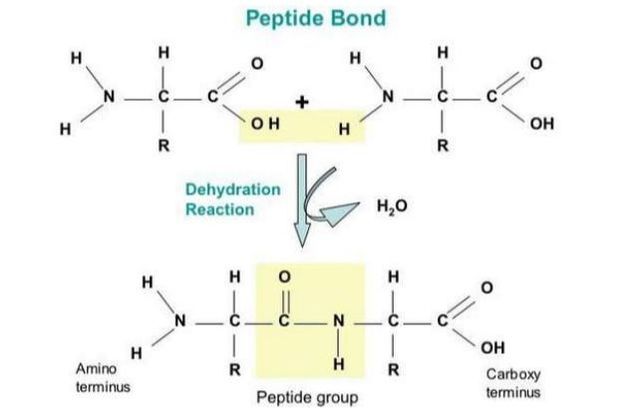
The condensation reaction involves the amine group (-NH2) of one amino acid and the carboxyl group (-COOH) of another. Water is removed as in all condensation reactions, and a new bond is formed between the two amino acids, called a peptide bond
Polypeptides can contain one protein some contain more
The condensation reaction involves the amine group (-NH2) of one amino acid and the carboxyl group (-COOH) of another. Water is removed as in all condensation reactions, and a new bond is formed between the two amino acids, called a peptide bond
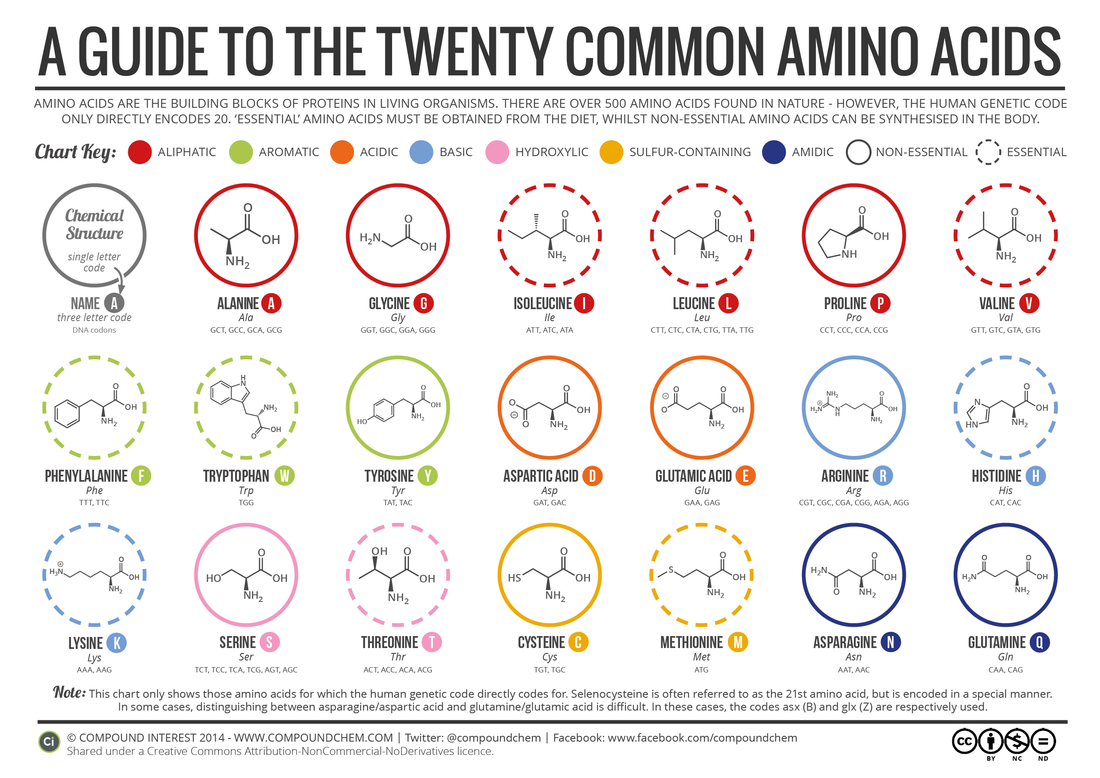
2.4.U.2 There are 20 different amino acids in polypeptides synthesized on ribosomes.
- State the number of amino acids used by living organisms to make polypeptides.
- Given an image of an amino acid, classify the amino acid chemical properties based on R group properties.
- Outline the role vitamin C plays in the conversion of proline to hydroxyproline.
2.4.U.3 Amino acids can be linked together in any sequence giving a huge range of possible polypeptides.
- Calculate the possible number of amino acid sequences given n number of amino acids.
Polypeptides are molecule consisting of many amino acids linked by peptide bonds. The polypeptides can contain any number of amino acids through chains of fewer than 20 amino acids are usually referred to as oligopeptides rather than polypeptides.
- insulin – small protein that contains two polypeptides, one with 21 amino acids and the other with 30
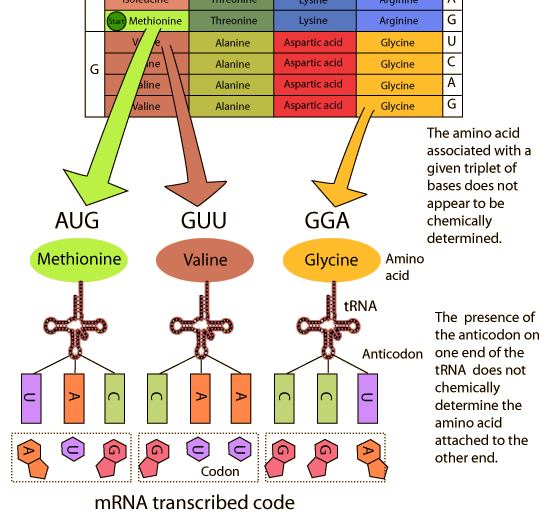
2.4.U.4 The amino acid sequence of polypeptides is coded for by genes.
- Outline the relationship between genes and polypeptides.
The number of amino acids sequences that could be produced is immense but living organisms only actually produce a small fraction of these. A typical cell produces polypeptides with thousands of different sequences and must store the information needed to do this- the amino acid of sequence of each polypeptide is stored in a coded form in the base sequence of a gene.
Some genes have other roles but most genes in a cell store the amino acid sequences of a polypeptide- using a genetic code to do this. Three bases of the gene code are needed to code for each amino acid in the polypeptide. The base sequence that actually codes for a polypeptide is known to molecular biologists as the open reading frame- one puzzle is that open reading frames only occupy a small proportion of the total DNA of a species.
Some genes have other roles but most genes in a cell store the amino acid sequences of a polypeptide- using a genetic code to do this. Three bases of the gene code are needed to code for each amino acid in the polypeptide. The base sequence that actually codes for a polypeptide is known to molecular biologists as the open reading frame- one puzzle is that open reading frames only occupy a small proportion of the total DNA of a species.
2.4.U.5 A protein may consist of a single polypeptide or more than one polypeptide linked together.
- Outline the structure and function of three example proteins composed of two or more polypeptides linked together.
Some proteins are single polypeptides but other are composed of two or more Polypeptides linked together
- a dipetide is a molecule consisting of two amino acids linked by a peptide bond
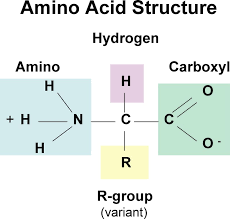
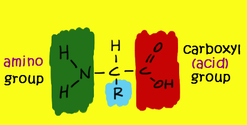
- amino acids that are linked together by ribosomes to make polypeptides all have some identical structural features – a carbon atom in the centre of the molecule is bonded to an amine group, a carboxyl group and a hydrogen atom
- carbon atom is also bonded to an R group = different in each amino acids
- amine groups and the carboxyl groups are used up in forming the petide bond – R group of the amino acids that give a polypeptide its character
- repertoire of R groups allows living organisms to make and use an amazingly wide range of proteins
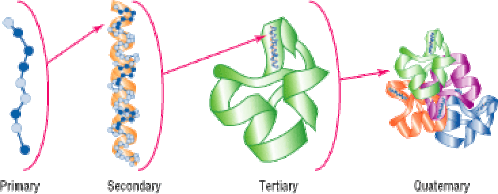
- certain proteins possess a fourth level of structural organisation called a quaternary structure
- quaternary structures are found in proteins that consist of more than one polypeptide chain linked together
- alternatively, proteins may have a quaternary structure if they include inorganic prosthetic groups as part of their structure
- not all proteins will have a quaternary structure – many proteins consist of a single polypeptide chain
2.4.U.6 The amino acid sequence determines the three-dimensional conformation of a protein.
- Contrast the structure of globular proteins with the structure of fibrous proteins.
- Describe the structure of membrane bound globular proteins.
The order of the amino acid sequence is called the primary structure and determines the way the chain will fold. The different amino acid sequences will fold into different configurations due to the chemical properties of the variable side chains. The amino acid sequences will commonly fold into two stable configurations, called secondary structures.
Alpha helices occur when the amino acid sequence folds into a coil / spiral arrangement. Beta-pleated sheets occur when the amino acid sequence adopts a directionally-oriented staggered strand conformation. Both α-helices and β-pleated sheets result from hydrogen bonds forming between non-adjacent amine and carboxyl groups
Where no secondary structure exists, the polypeptide chain will form a random coil conformation of a protein is its three dimensional structure.
The overall three-dimensional configuration of the protein is referred to as the tertiary structure of the protein The tertiary structure of a polypeptide chain will be determined by the interactions between the variable side chains
These interactions may include hydrogen bonds, disulphide bridges, ionic interactions, polar associations, etc.
Alpha helices occur when the amino acid sequence folds into a coil / spiral arrangement. Beta-pleated sheets occur when the amino acid sequence adopts a directionally-oriented staggered strand conformation. Both α-helices and β-pleated sheets result from hydrogen bonds forming between non-adjacent amine and carboxyl groups
Where no secondary structure exists, the polypeptide chain will form a random coil conformation of a protein is its three dimensional structure.
The overall three-dimensional configuration of the protein is referred to as the tertiary structure of the protein The tertiary structure of a polypeptide chain will be determined by the interactions between the variable side chains
These interactions may include hydrogen bonds, disulphide bridges, ionic interactions, polar associations, etc.
- determined by the amino acid sequence of a protein and its constituent polypeptides
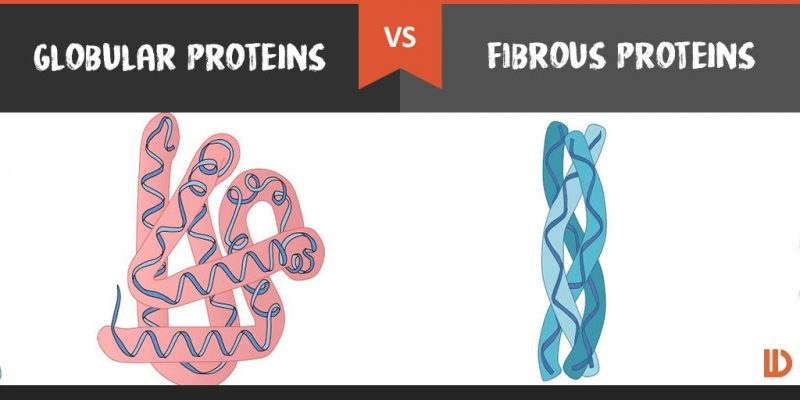
- fibrous proteins such a collagen are elongated usually with a repeating structure
- many proteins are globular with an intericate shape that oftern includes parts that are helical or sheet like
- amino acids are added one by one to form a polypeptide
- they are always added in the same sequence to make a particular polypeptide
- in globular proteins the polypeptides gradually fold up as they are made to develop the final conformation= this is stabilized by bonds between the R groups of the amino acids that have been brought together by the folding
- globular proteins that are soluble in water- there are hydrophilic R groups on the outside of the molecules and there are usually hydrophobic groups on the inside
- in globular membrane proteins there are regions with hydrophobic R groups on the outside of the molecules which are attracted to the hydrophobic centre of the membrane
- in fibrous proteins the amino acid sequence prevents folding up and ensures that the chain of amino acids remains in an elongated form.
2.4.U.7 Living organisms synthesize many different proteins with a wide range of functions.
- Contrast the generalized function of globular proteins with generalized function of fibrous proteins.
- List ten functions of proteins in a cell or organism.
- Describe the function of enzyme proteins.
- Describe the function of hormone proteins.
- Describe the function of immunoglobulin proteins.
- Describe the function of pigment proteins.
- Describe the function of structural proteins
- Catalysis - there are thousdans of different enzymes to catalyse specific chemical reactions within the cell oroutside it
- Muscle contraction - actin and myosin together cause the muscle contractions used in locomotion and transport around the body
- Cytoskeletons - tubulin is the subunit of microtubules that give animal cells their shape and pull on chromosomes during mitosis
- Tensil strengthening - fibrous proteins give tensile strength needed in skin, tendons, ligaments and blood vessel walls
- Blood clotting - plasma proteins act as clotting factors that cause blood to turn from a liquid to a gel in wounds
- Transport of nutrients and gases - proteins in blood help transport oxygen, carbon dioxide, iron and lipids
- Cell adhesion - membrane proteins cause adjacent animal cells to stick to each other within tissue
- Membrane transport - membrane proteins are used for facilitated diffusion and active transport, and also for elecgtron transport during cell respiratoi and photosynthesis
- Hormones - some such as insulin FSH and LH are proteins, but hormones are chemicall yver diverse.
- Receptors - binding sites in membranes and cytoplasm for hormones, neurotransmitters, tastes and smells, and also receptors for light in the eye and in plants.
- Packing of DNA - histones are associated with DNA in eukaryotes and help chromosomes to condense during mitosis
- Immunity - this is the most diverse group of proteins, as cells cab make huge numbers of different antibodies.
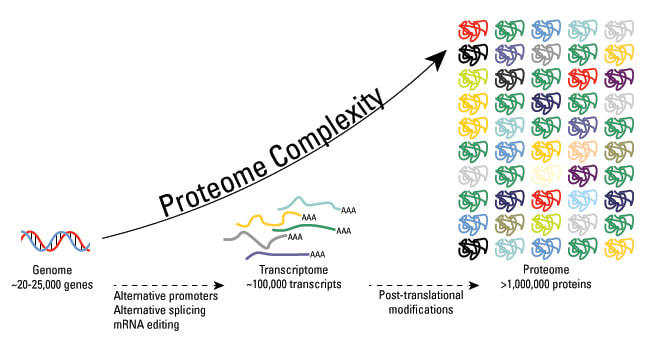
2.4.U.8 Every individual has a unique proteome.
- Define proteome.
- Contrast proteome with genome.
The proteome is the totality of proteins expressed within a cell, tissue or organism at a certain time. The proteome of any given individual will be unique, as protein expression patterns are determined by an individual’s genes
- all of the proteins produced by a cell, a tissue or an organism .
- compared to the genome is all of the genes of a cell, a tissue or an organism
- to find out how many different proteins are being produced mixtures of proteins are extracted from a sample and are then separated by gel electrophoresis
- this is to indentify whether or not a particular protein is present, antibodies to the protein that have been linked to a fluorescent marker can be used – if the cell fluoresces the protein is present
- BUT the genome of an organism is fixed,the proteome is variable because different cells in an organism make different proteins
- even in a single cell the proteins that are made vary over time depending on the cells activities – the proteome therefore reveals what is actually happening in an organism, not what potentially could happen
- within a species there are strong similarities in the proteome of all individuals, but also differences
- proteome of each individual is unique partly because of differences of activity but also because of differences in the amino acid sequence of proteins- with the possible exception of identical twins, none of us have identical proteins, so each of us has a unique proteome, even the proteome of identical twins can become different with age.
Applications
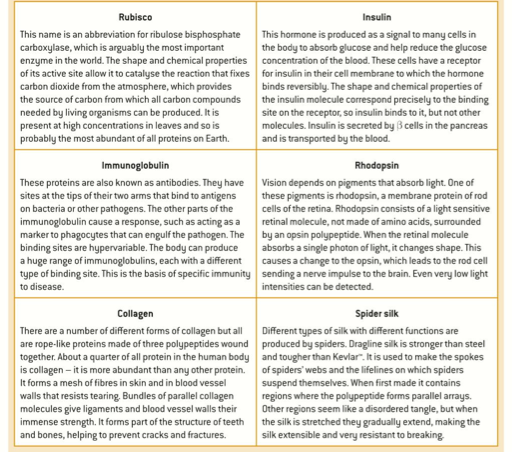
2.4.A.1 Rubisco, insulin, immunoglobulins, rhodopsin, collagen and spider silk as examples of the range of protein functions.
- State the function of each of the following proteins: rubisco, insulin, immunoglobulin, rhodopsin. collagen, spider silk, actin, myosin, casein, hemoglobin, acetylcholine receptor, oxytocin, prolactin, ferritin, billirubin, fibrinogen, transferrin and albumin.
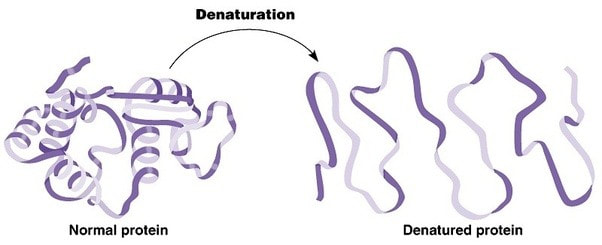
2.4.A.2 Denaturation of proteins by heat or by deviation of pH from the optimum.
- Define denaturation.
- Outline the effect of heat and pH on protein structure.
Denaturation of proteins involves the disruption and possible destruction of both the secondary and tertiary structures. Since denaturation reactions are not strong enough to break the peptide bonds, the primary structure (sequence of amino acids) remains the same after a denaturation process. Denaturation disrupts the normal alpha-helix and beta sheets in a protein and uncoils it into a random shape
Denaturation of proteins can usually be caused by two key conditions – temperature and pH
Temperature
Denaturation of proteins can usually be caused by two key conditions – temperature and pH
Temperature
- High levels of thermal energy may disrupt the hydrogen bonds that hold the protein together
- As these bonds are broken, the protein will begin to unfold and lose its capacity to function as intended
- Temperatures at which proteins denature may vary, but most human proteins function optimally at body temperature (~37ºC)
- Amino acids are zwitterions, neutral molecules possessing both negatively (COO–) and positively (NH3+) charged regions
- Changing the pH will alter the charge of the protein, which in turn will alter protein solubility and overall shape
- All proteins have an optimal pH which is dependent on the environment in which it functions (e.g. stomach proteins require an acidic environment to operate, whereas blood proteins function best at a neutral pH)
Skills:
2.4.S.1 Drawing molecular diagrams to show the formation of a peptide bond.
- Draw peptide bond formation in a condensation reactions.
Peptide bonds are formed between the amine and carboxylic acid groups of adjacent amino acids
The amine group loses a hydrogen atom (H) and the carboxylic acid loses a hydroxyl (OH) – this forms water (H2O)
The amine group loses a hydrogen atom (H) and the carboxylic acid loses a hydroxyl (OH) – this forms water (H2O)
Key Terms:
|
amino acids
ribosomes fibrous proteins genome spider silk oxytocin transferrin carboxyl groups |
polypeptide
R-groups hormone proteins rubisco actin prolactin fibrinogen scurvy |
peptide bonds
genes immunoglobulin proteins insulin myosin acetylcholine receptor albumin integrin |
condensation
globular proteins pigment proteins rhodopsin casein ferritin denature catalysis |
dipeptide
oligopeptides proteome collagen hemoglobin bilirubin amine groups gel electrophoresis |
Class Assignment:
Protein Rules
Modeling Protein Lab
Protein Denaturation Lab
Amino Acids
Topic 2.4 Review
Topic 2.4 Kahoot Review Quiz
Protein Rules
Modeling Protein Lab
Protein Denaturation Lab
Amino Acids
Topic 2.4 Review
Topic 2.4 Kahoot Review Quiz
Powerpoint and Notes on Topic 2.4 by Chris Payne
Your browser does not support viewing this document. Click here to download the document.
Your browser does not support viewing this document. Click here to download the document.
Correct use of terminology is a key skill in Biology. It is essential to use key terms correctly when communicating your understanding, particularly in assessments. Use the quizlet flashcards or other tools such as learn, scatter, space race, speller and test to help you master the vocabulary.
,
Helpful Links:
The Macromolecules of Life
BBC Bitesize
Protein Denature McGraw Hill
Biomolecules: Proteins
Amino Acid and Peptide Bond Animation
Life Cycle of a Protein
Heat Changes a Protein Structure
Protein Secretion
Acideroids game
Amino acids and proteins from John Kyrk
Life Cycle of a Protein from Sumanas
Making polypeptides from John Kyrk
Protein Structures and Protein Folding by John Gianni
In the News:
U of A professors research link between proteins and Alzheimer’s - Global News
Protein 'can stop viruses developing' - BBC News, 19 October 2017
Helpful Links:
The Macromolecules of Life
BBC Bitesize
Protein Denature McGraw Hill
Biomolecules: Proteins
Amino Acid and Peptide Bond Animation
Life Cycle of a Protein
Heat Changes a Protein Structure
Protein Secretion
Acideroids game
Amino acids and proteins from John Kyrk
Life Cycle of a Protein from Sumanas
Making polypeptides from John Kyrk
Protein Structures and Protein Folding by John Gianni
In the News:
U of A professors research link between proteins and Alzheimer’s - Global News
Protein 'can stop viruses developing' - BBC News, 19 October 2017
TOK:
- Development of some techniques benefits particular human populations more than others. For example, the development of lactose-free milk available in Europe and North America would have greater benefit in Africa/ Asia where lactose intolerance is more prevalent. The development of techniques requires financial investment. Should knowledge be shared when techniques developed in one part of the world are more applicable in another?
Video Clips:
Paul Andersen explains the structure and importance of proteins. He describes how proteins are created from amino acids connected by dehydration synthesis. He shows the importance of chemical properties in the R-groups of individual amino acids in the polypeptide. He explains the four levels of protein folding and gives you an opportunity to fold proteins of your own using the game Foldi
Professor Dave explains how everyone has heard of proteins. What are they on the molecular level? They're polymers of amino acids, of course. They make up most of your body, so we have to understand their structure very well! Check this out to learn the hierarchy of protein structure so that we can later learn all about what different types of proteins can do.
Big leaps in our understanding of protein folding can open doors to new protein-based medicines and materials--designed from the ground up.
For 50 years, the "protein folding problem" has been a major mystery. How does a miniature string-like chemical -- the protein molecule - encode the functions of living organisms: how our muscles exert force, how our immune systems reject pathogens, how our eyes see our surroundings, how plants convert solar energy, and all the rest. Huge progress is being made. Moreover, these amazing nano-machines could play important roles in health and disease and commerce in the future.
Protein Structure and Protein Denaturation HD Animation