4.3 carbon cycle
In the Carbon Cycle we will look at how carbon is one of the most important elements that are recycled in an ecosystem. We will see how Inorganic carbon dioxide in the atmosphere is trapped or fixed as organic carbon compounds during photosynthesis.
This unit will last 3 days
This unit will last 3 days
Essential idea:
- Continued availability of carbon in ecosystems depends on carbon cycling.
Nature of science:
- Making accurate, quantitative measurements—it is important to obtain reliable data on the concentration of carbon dioxide and methane in the atmosphere. (3.1)
- Explain why accurate measurements of CO2 and methane in the atmosphere are important.
- Outline how data on concentration of atmospheric CO2 and methane are collected.
- Explain why accurate measurements of CO2 and methane in the atmosphere are important.
Understanding
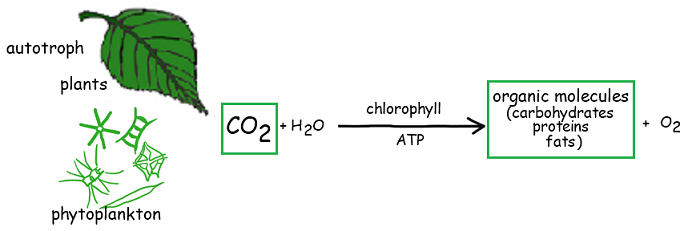
4.3.U1 Autotrophs convert carbon dioxide into carbohydrates and other carbon compounds
- State the role of photosynthesis in the carbon cycle.
Autotrophs absorb carbon dioxide from the atmosphere and convert it into carbohydrates, lipids, and all the other carbon compounds that they require. This has the effect of reducing the carbon dioxide concentration of the atmosphere
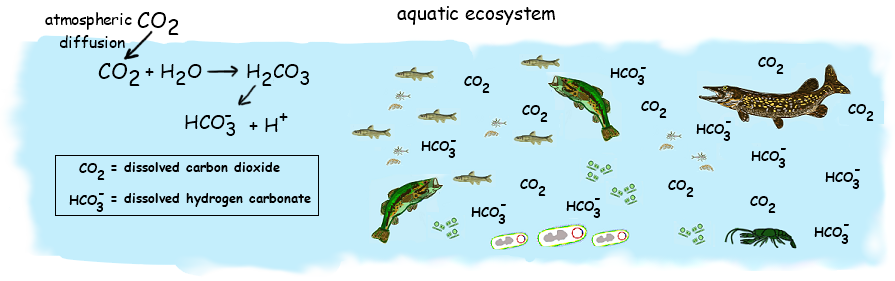
4.3.U2 In aquatic ecosystems carbon is present as dissolved carbon dioxide and hydrogencarbonate ions.
- Outline the process that converts CO2 to hydrogen carbonate ion in water, leading to a reduction of the pH in the water.
Carbon dioxide dissolves in water and some of it will remain as a dissolved gas, however the remainder will combine with water to form carbonic acid (CO2 + H2O ⇄ H2CO3)
- Carbon dioxide is soluble in water – can remain in water as dissolved gas or can combine with water to form carbonic acid (H2CO3)
- Carbonic acid can dissociate to form hydrogen and hydrogen carbonate ions (H+ and HCO3-) -> explains how CO2 can reduce the pH of water
- Dissolved CO2 and hydrogen carbonate ions are absorbed by aquatic plants / other autotrophs that live in water
- Use them to make carbohydrates and other carbon compounds
4.3.U3 Carbon dioxide diffuses from the atmosphere or water into autotrophs.
- State that in diffusion, molecules move from an area of higher concentration to an area of lower concentration.
Autotrophs, such as all plants and algae, convert inorganic carbon dioxide into organic compounds via photosynthesis. These organic compounds include the carbohydrates, lipids and proteins required by the organism for survivalA
Autotrophs use CO2 in the production of carbon compounds by photosynthesis or other processes. – Reduces the concentration of CO2 inside autotrophs and sets up a concentration gradient between cells in autotrophs and the air/water around
Autotrophs use CO2 in the production of carbon compounds by photosynthesis or other processes. – Reduces the concentration of CO2 inside autotrophs and sets up a concentration gradient between cells in autotrophs and the air/water around
- CO2 diffuses from the atmosphere or water into autotrophs
- In land plants – occurs in the stomata in the underside of leaves
- Aquatic plants – entire surface of leaves / stems usually permeable to CO2 (diffusion can be through any part of the plant)
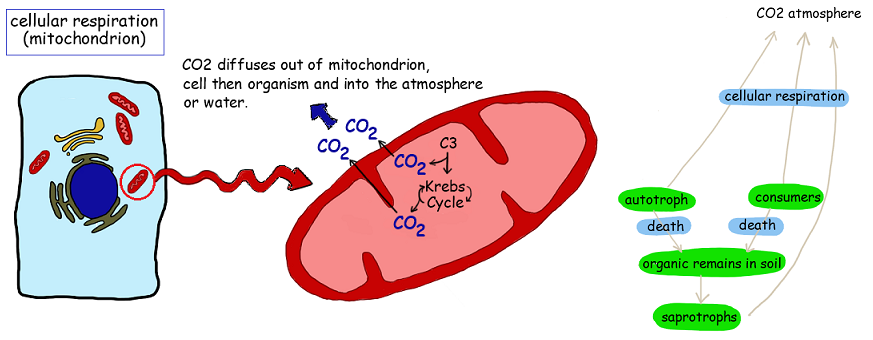
4.3.U4 Carbon dioxide is produced by respiration and diffuses out of organisms into water or the atmosphere
- State that carbon dioxide is a waste product of aerobic cellular respiration.
- State that carbon dioxide diffuses out of cells into the atmosphere or water.
All organisms may produce the chemical energy (ATP) required to power metabolic processes via the process of cell respiration
- CO2 is a waste product of aerobic cell respiration – produced in all cells that carry out aerobic cell respiration. Can be grouped according to trophic level:
- Non-photosynthetic cells in produces (eg root cells)
- Animal cells
- Saprotrophs (eg fungi that decompose dead organic matter)
- CO2 produced by respiration diffuses out of cells and passes into the atmosphere or water that surrounds the organism
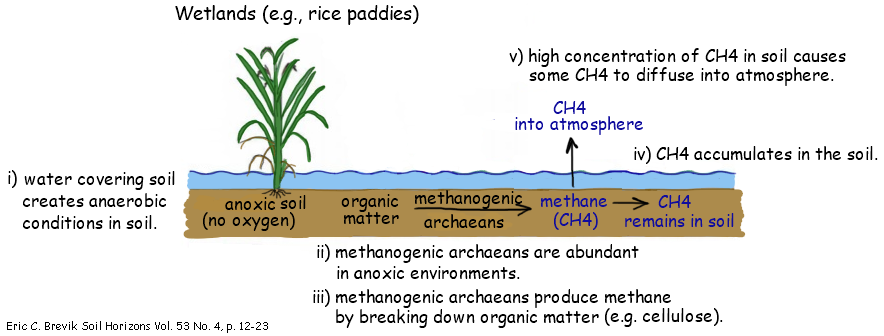
4.3.U5 Methane is produced from organic matter in anaerobic conditions by methanogenic archaeans and some diffuses into the atmosphere or accumulates in the ground.
- Outline the role of methanogenic archaea in the transformation of organic material into methane
Methane is produced widely in anaerobic environments – it is a waste product of a type of anaerobic respiration
3 groups of anaerobic prokaryotes involved:
Archaeans in this group are methanogenic – they carry out methanogenesis in many anaerobic environments:
Landfill sites where organic matter is in wastes that have been buried
Some methane produced by archaeans is released into the atmosphere
Methane produced from organic waste in anaerobic digesters is not allowed to escape is instead burned as a fuel
3 groups of anaerobic prokaryotes involved:
- Bacteria that convert organic matter into mixture of organic acids, alcohol, hydrogen, and carbon dioxide
- Bacteria that use organic acids and alcohol to produce acetate, carbon dioxide, and hydrogen
- Archaeans that produce methane from carbon dioxide, hydrogen and acetate do this by two chemical reactions:
- CO2 + 4H2 -> CH4 + 2H20
- CH3COOH -> CH4 + CO2
Archaeans in this group are methanogenic – they carry out methanogenesis in many anaerobic environments:
- Mud along shores / in bed of lakes
- Swamps, mires, mangrove forests and other wetlands where soil or peat deposits are waterlogged
- Guts of termites and of ruminant mammals like cattle / sheep
Landfill sites where organic matter is in wastes that have been buried
Some methane produced by archaeans is released into the atmosphere
Methane produced from organic waste in anaerobic digesters is not allowed to escape is instead burned as a fuel
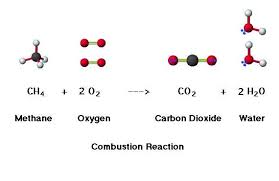
4.3.U6 Methane is oxidized to carbon dioxide and water in the atmosphere.
- State that methane is oxidized to carbon dioxide in the atmosphere.
When methane is released into the atmosphere as a result of anaerobic reactions, it only persists for ~12 years. Methane will be naturally oxidised to form carbon dioxide and water (CH4 + 2 O2 → CO2 + 2 H2O)
- Monatomic oxygen and highly reactive hydroxyl radicals are involved in methane oxidation
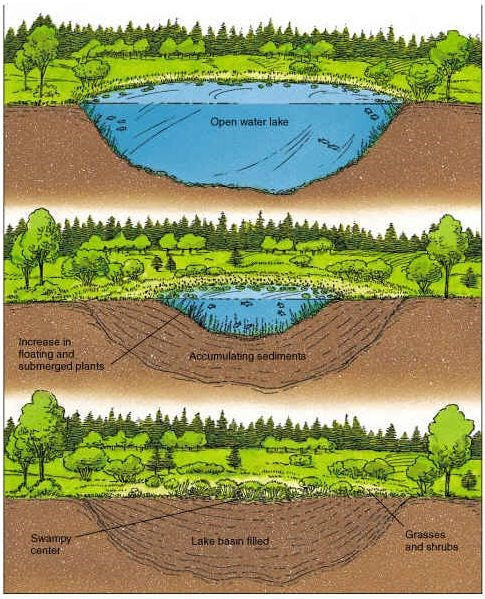
4.3.U7 Peat forms when organic matter is not fully decomposed because of acidic and/or anaerobic conditions in waterlogged soils.
- Define peat.
- Outline formation of peat.
In many soils, saprotrophic bacteria and fungi will decompose dead organisms and return nutrients to the soil for cycling. This decomposition process requires oxygen (cell respiration is required to fuel digestive reactions)
- Peat forms when organic matter is not fully decomposed because of anaerobic conditions in waterlogged soils
large quantities of organic matter accumulate and become compressed to form a dark brown acidic material called peat - Dead leaves from plants is eventually digested by saprotrophic bacteria and fungi
- Saprotrophs obtain oxygen for respiration from airspaces in soil
- In some environments, water cannot drain out of soils – become waterlogged and anaerobic
- Saprotrophs can’t thrive – dead organic matter isn’t fully decomposed
- Acidic conditions tend to develop – further inhibiting saprotrophs and methanogens that might break down the organic matter
- Large quantities of partially decomposed organic matter have accumulated in some ecosystems and become compressed to form a dark brown acidic material called peat
- Conditions go from being aerobic to being anaerobic.
- Becomes more and more acidic -> methanogens cannot work any longer. (The methanogens are releasing carbon dioxide which is what causes the water to become more acidic)
4.3.U8 Partially decomposed organic matter from past geological eras was converted either into coal or into oil and gas that accumulate in porous rocks.
- Outline formation of coal.
- Outline formation of oil and natural gas.
Oil (i.e. petroleum) and natural gas form as the result of the decay of marine organisms on the ocean floor
- Carbon / some carbon compounds are chemically stable and can remain unchanged in rocks for hundreds of millions of years
- Large deposits of carbon from past geological eras – a result of incomplete decomposition of organic matter. Burial in sediments became rock
- Coal formed when deposits of peat are buried under other sediments. Peat is compressed/heated – turns into coal.
- Oil and natural gas formed in the mud at bottom of seas and lakes. Conditions usually anaerobic so decomposition is often incomplete. More mud/other sediments are deposited, partially decomposed matter is compressed/heated. Chemical changes occur – produce complex mixtures of liquid carbon compounds or gases. -> crude oil and natural gas. Methane forms the largest part of natural gas.
4.3.U9 Carbon dioxide is produced by the combustion of biomass and fossilized organic matter.
- Define combustion.
- State the products of a combustion reaction.
- State sources of fuel for a combustion reaction.
When organic compounds rich in hydrocarbons are heated in the presence of oxygen, they undergo a combustion reaction. This reaction is exergonic (produces energy) and releases carbon dioxide and water as by-products
The carbon dioxide is typically released into the atmosphere, increasing the concentration of the gas in the ai
The carbon dioxide is typically released into the atmosphere, increasing the concentration of the gas in the ai
- If organic matter is heated to ignition temp. in the pressure of oxygen – will set light and burn. -> Oxidation reactions called combustion
- Products of combustion are CO2 and H2O
- Biomass: total mass of a group of organisms (dry mass)
- Units of grams/meter squared (g/m^2) = g m^-2 -> land and terrestrial; g m^3 -> aquatic
- One way that carbon enters the atmosphere
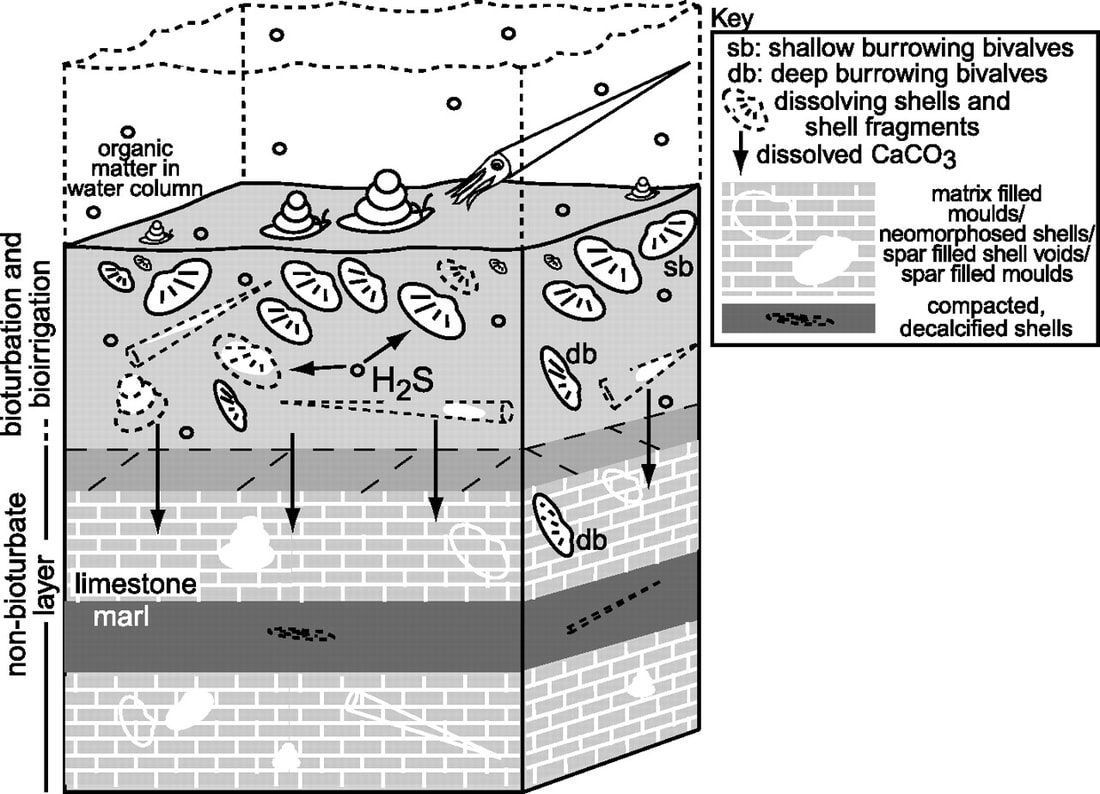
4.3.U10 Animals such as reef-building corals and mollusca have hard parts that are composed of calcium carbonate and can become fossilized in limestone.
- State that hard shells, such as in mollusk and coral, are made of calcium carbonate.
Some animals like Molusca have hard body parts composed of calcium carbonate (CaCO3).
Hard corals that build reefs (exoskeletons by secreting calcium carbonate)
When these animals die, soft parts are decomposed quickly. IN acid conditions, calcium carbonate dissolves but in neutral/alkaline conditions, it is stable and deposits of it from hard animal parts can form on the sea bed.. In shallow tropical seas calcium carbonate is deposited by precipitation in the water – result is limestone rock.
Hard corals that build reefs (exoskeletons by secreting calcium carbonate)
When these animals die, soft parts are decomposed quickly. IN acid conditions, calcium carbonate dissolves but in neutral/alkaline conditions, it is stable and deposits of it from hard animal parts can form on the sea bed.. In shallow tropical seas calcium carbonate is deposited by precipitation in the water – result is limestone rock.
Application
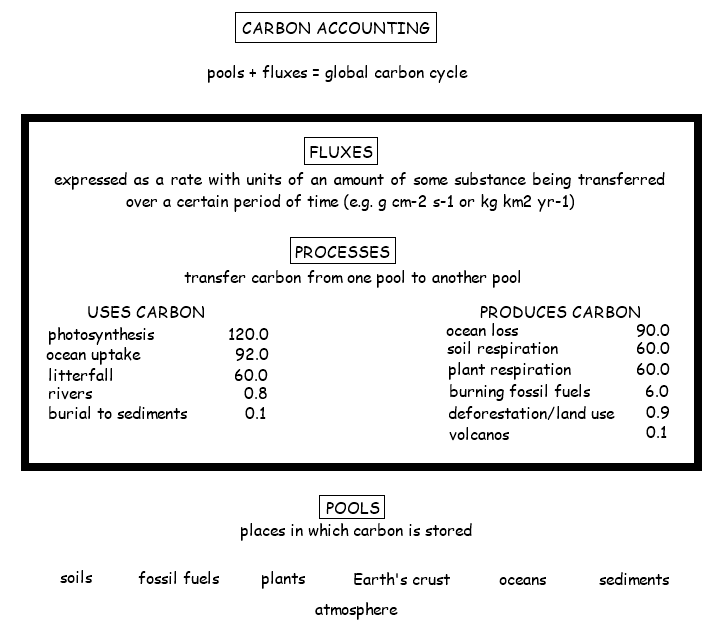
4.3.S1 Estimation of carbon fluxes due to processes in the carbon cycle. (Carbon fluxes should be measured in gigatonnes.)
- List seven flux processes in the carbon cycle.
- State the unit of measure for carbon flux values.
Carbon fluxes describe the rate of exchange of carbon between the various carbon sinks / reservoirs. There are four main carbon sinks – lithosphere (earth's crust), hydrosphere (oceans), atmosphere (air), biosphere (organisms)
The rate at which carbon is exchanged between these reservoirs depends on the conversion processes involved:
The rate at which carbon is exchanged between these reservoirs depends on the conversion processes involved:
- Photosynthesis – removes carbon dioxide from the atmosphere and fixes it in producers as organic compounds
- Respiration – releases carbon dioxide into the atmosphere when organic compounds are digested in living organisms
- Decomposition – releases carbon products into the air or sediment when organic matter is recycled after death of an organism
- Gaseous dissolution – the exchange of carbon gases between the ocean and atmosphere
- Lithification – the compaction of carbon-containing sediments into fossils and rocks within the Earth’s crust (e.g. limestone)
- Combustion – releases carbon gases when organic hydrocarbons (coal, oil and gas) are burned as a fuel source
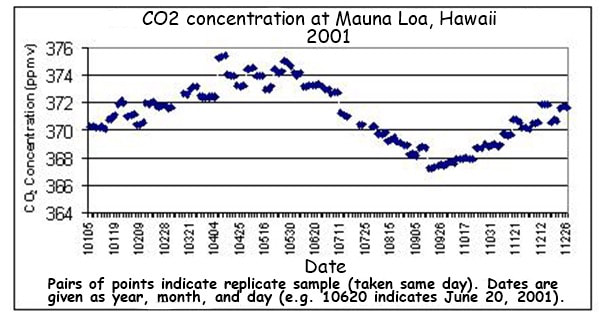
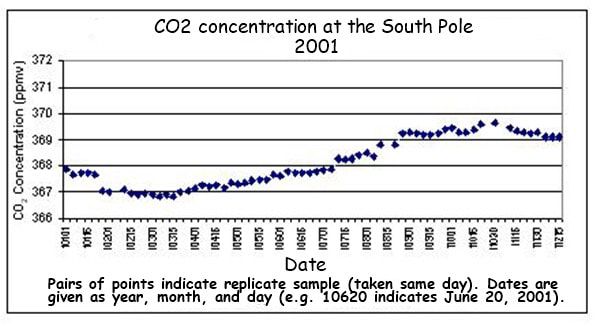
4.3.S2 Analysis of data from air monitoring stations to explain annual fluctuations
- Sketch a graph of the annual fluctuation in atmospheric carbon dioxide concentration.
- Explain the annual fluctuation in atmospheric carbon dioxide concentration in the northern hemisphere.
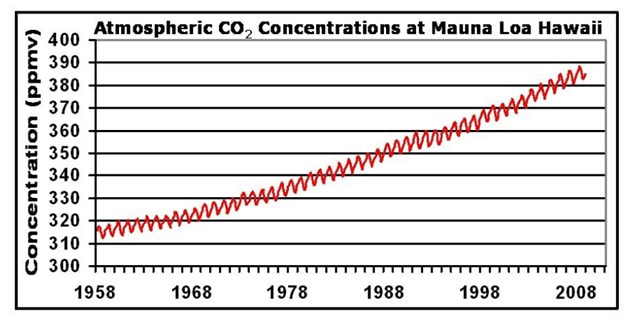
Atmospheric CO2 concentrations have been measured at the Mauna Loa Observatory (in Hawaii) since 1958 by Charles Keeling. From these continuous and regular measurements a clear pattern of carbon flux can be seen:
- CO2 levels fluctuate annually (lower in the summer months when long days and more light increase photosynthetic rates)
- Global CO2 trends will conform to northern hemisphere patterns as it contains more of the planet’s land mass (i.e. more trees)
- CO2 levels are steadily increasing year on year since the industrial revolution (due to increased burning of fossil fuels)
- Atmospheric CO2 levels are currently at the highest levels recorded since measurements began
Data is now being regularly collected at a variety of field stations globally, using standardised measurement techniques
Carbon data can be plotted and analysed using the online database at CDIAC (Carbon Dioxide Information Analysis Centre)
How to use the CDIAC database:
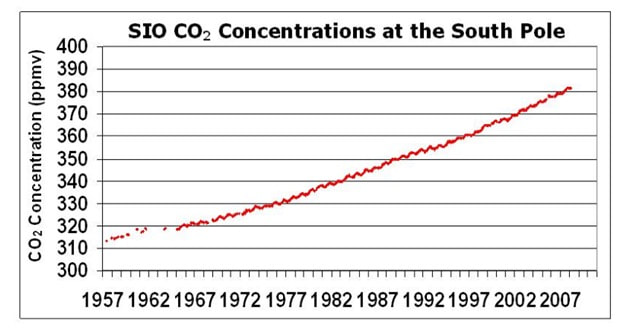
- All stations show a clear upward trend in atmospheric CO2 concentrations year on year, with annual fluctuations
- Different monitoring stations may have slightly different trends due to seasonal variations and the distribution of local vegetation
Carbon data can be plotted and analysed using the online database at CDIAC (Carbon Dioxide Information Analysis Centre)
- This website stores data on atmospheric CO2 levels, which can be imported into an Excel spreadsheet in order to graph
How to use the CDIAC database:
- Access the CDIAC website (click on the link to redirect)
- Click on ‘Atmospheric Trace Gases and Aerosols’ (under ‘Data' tab at top of page)
- Select ‘Carbon dioxide’ from the list of greenhouse gases
- Choose a monitoring station / network (e.g. Scripps Institution of Oceanography Network)
- Download data from a particular site (e.g. South Pole, Antarctica)
- Paste data of interest into an Excel spreadsheet to produce a graphical display (e.g. Jan 2000 – Dec 2007)
Skill
4.3.S1 Construct a diagram of the carbon cycle.
- Draw a diagram of the terrestrial carbon cycle.
- Draw a diagram of the aquatic carbon cycle.
- Define pool and flux.
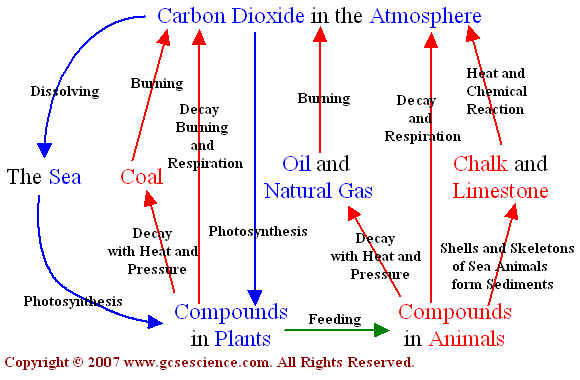
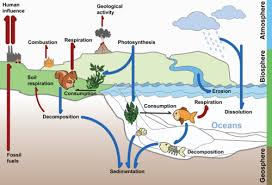
The carbon cycle is a biogeochemical cycle whereby carbon is exchanged between the different spheres of the Earth. The four spheres are the atmosphere (air), lithosphere (ground), hydrosphere (water / oceans) and biosphere (living things). Carbon is exchanged between a variety of forms, including:
Atmospheric gases – mainly carbon dioxide (CO2), but also methane (CH4)
Oceanic carbonates – including bicarbonates dissolved in the water and calcium carbonate in corals and shells
As organic materials – including the carbohydrates, lipids and proteins found in all living things
As non-living remains – such as detritus and fossil fuels
Details include
Atmospheric gases – mainly carbon dioxide (CO2), but also methane (CH4)
Oceanic carbonates – including bicarbonates dissolved in the water and calcium carbonate in corals and shells
As organic materials – including the carbohydrates, lipids and proteins found in all living things
As non-living remains – such as detritus and fossil fuels
Details include
- interaction of living organisms and the biosphere through:
- photosynthesis - atmospheric carbon dioxide --> organisms
- cell respiration - organisms --> atmospheric carbon dioxide
- fossilization - carbon-containing molecules --> fossil fuels
- combustion - carbon-containing organisms & fossil fuels --> atmospheric carbon dioxide
- sedimentation - carbon-containing molecules --> mineral deposits
- volcanoes - carbon-containing mineral deposits --> atmospheric carbon dioxide
- recall of quantitative data is not required
Key Terms
|
carbon
glucose carbon dioxide peat carbon fixation monoatomic oxygen fossilized organic matter |
hydrogen carbonate
methanogenesis organic acids acetate Archaeans combustion sedimentary rock |
methanogenic
carbohydrate fossil fuels hydrocarbons carbonic acid hydroxyl radicals limestone coal |
carbonates
excretion cycling limestone Ruminant mammals peat carbon flux |
autotrophs
combustion methane biogas landfill site coal photosynthesis |
Classroom Material
Carbon Cycle activity
Carbon Cycle Flash Cards
Carbon Cycle Activity Cards
BioFact Sheet - Nutrient Cycles
Global Carbon Fluctuations Simulations
Topic 4.3 Review Guide
Carbon Cycle activity
Carbon Cycle Flash Cards
Carbon Cycle Activity Cards
BioFact Sheet - Nutrient Cycles
Global Carbon Fluctuations Simulations
Topic 4.3 Review Guide
PowerPoint and Study Guide on Topic 4.3 by Chris Payne
Your browser does not support viewing this document. Click here to download the document.
Your browser does not support viewing this document. Click here to download the document.
Correct use of terminology is a key skill in Biology. It is essential to use key terms correctly when communicating your understanding, particularly in assessments. Use the quizlet flashcards or other tools such as learn, scatter, space race, speller and test to help you master the vocabulary.
Topic 4.3 Review video by Mr. Leonard
Useful Resources
Global carbon cycle tutorial from FreemanLifewire
Narrated tutorial from Sumanas
Good simple interactive pathways of carbon from KScience, and another from windows2universe
Measuring Current CO2 levels
Current atmospheric CO2 is measured by hundreds of field stations around the globe, inputting data into the NOAA databases.
Use these CO2 data to plot trends and annual cycles with a spreadsheet.
Check out CO2Now to see graphics of the most recent data and trends.
Measuring Historical CO2 levels
This resource from Allianz(?) outlines how ice-core data is collected and analysed
The NOAA Satellite and Information Service has databases for all the different methods used to gather historical CO2 data. You could also use the Vostok data.
Ocean acidification – the other CO2 problem:
Find out more about measuring ocean CO2 data from the CDIAC. The National Resources Defense Council outlines the harmful effects of ocean acidification.
Global carbon cycle tutorial from FreemanLifewire
Narrated tutorial from Sumanas
Good simple interactive pathways of carbon from KScience, and another from windows2universe
Measuring Current CO2 levels
Current atmospheric CO2 is measured by hundreds of field stations around the globe, inputting data into the NOAA databases.
Use these CO2 data to plot trends and annual cycles with a spreadsheet.
Check out CO2Now to see graphics of the most recent data and trends.
Measuring Historical CO2 levels
This resource from Allianz(?) outlines how ice-core data is collected and analysed
The NOAA Satellite and Information Service has databases for all the different methods used to gather historical CO2 data. You could also use the Vostok data.
Ocean acidification – the other CO2 problem:
Find out more about measuring ocean CO2 data from the CDIAC. The National Resources Defense Council outlines the harmful effects of ocean acidification.
In The News
In the ocean's twilight zone, tiny organisms may have giant effect on Earth's carbon cycle - Science Daily July 2018
Arctic carbon cycle is speeding up - NASA, August 2018
Giant icebergs play 'major role' in ocean carbon cycle - BBC January 2016
Are methane seeps in the Arctic slowing global warming - Science, May 2017
In the ocean's twilight zone, tiny organisms may have giant effect on Earth's carbon cycle - Science Daily July 2018
Arctic carbon cycle is speeding up - NASA, August 2018
Giant icebergs play 'major role' in ocean carbon cycle - BBC January 2016
Are methane seeps in the Arctic slowing global warming - Science, May 2017
Videos:
Carbon is the basic building block of life, and these unique atoms are found everywhere on Earth. Carbon makes up Earth's plants and animals, and is also stored in the ocean, the atmosphere, and the crust of the planet. A carbon atom could spend millions of years moving through Earth in a complex cycle. This conceptual animation provides an illustration of the various parts of the Carbon cycle. Purple arrows indicate the uptake of Carbon; yellow arrows indicate the release of Carbon.
Hank takes us on a tour of the The Global Carbon Cycle and how it all works. From Carbon Fixation to Redox Reactions, it's all contained within!
In this video Paul Andersen explains how biogeochemical cycles move required nutrients through the abiotic and biotic spheres on our planet. Matter on the Earth is conserved so producers must receive required nutrients through the water cycle, carbon cycle, nitrogen cycle, phosphorus cycle, and sulfur cycle.
Hank introduces us to biogeochemical cycles by describing his two favorites: carbon and water. The hydrologic cycle describes how water moves on, above, and below the surface of the Earth, driven by energy supplied by the sun and wind. The carbon cycle does the same... for carbon!
Where are we now? In a time of need for resilience and inventive solutions. Click here for excerpts from their post-carbon reader.
This video clip from NOAA has seasonal sources and sinks of CO2: