topic 6 enzymes

Enzymes are biological molecules (typically proteins) that significantly speed up the rate of virtually all of the chemical reactions that take place within cells.
They are vital for life and serve a wide range of important functions in the body, such as aiding in digestion and metabolism.
Some enzymes help break large molecules into smaller pieces that are more easily absorbed by the body. Other enzymes help bind two molecules together to produce a new molecule. Enzymes are highly selective catalysts, meaning that each enzyme only speeds up a specific reaction
They are vital for life and serve a wide range of important functions in the body, such as aiding in digestion and metabolism.
Some enzymes help break large molecules into smaller pieces that are more easily absorbed by the body. Other enzymes help bind two molecules together to produce a new molecule. Enzymes are highly selective catalysts, meaning that each enzyme only speeds up a specific reaction
Describe the structure of an enzyme
- Describe: Give a detailed account or picture of a situation, event, pattern or process.
The structure of an enzyme is crucially important for its function. The reaction that an enzyme catalyses occurs on the active site, which is the area of the protein in which the substrate can bind and the chemical reaction can take place. The active site is usually on the surface of the protein so it can be easily accessed, and usually has a highly specific structure that allows it to bind its substrate and carry out its catalytic activity. This structure is determined by the different side chains and modifications present in amino acids in the chain, and the resulting final structure the enzyme adopts. Usually, each enzyme only has one active site that can bind one substrate or class of substrates.
Explain that enzymes have an active site to which specific substrates bind.
- Explain: Give a detailed account including reasons or causes.
An enzyme is a globular protein which acts as a biological catalyst by speeding up the rate of a chemical reaction. Enzymes are not changed or consumed by the reactions they catalyse and thus can be reused. Enzymes are typically named after the molecules they react with (called the substrate) and end with the suffix ‘-ase’. The active site is the region on the surface of the enzyme which binds to the substrate molecule. The active site and the substrate complement each other in terms of both shape and chemical properties. Only a specific substrate is capable of binding to a particular enzyme’s active site
- work as globular proteins that work as catalysts = speed up chemical reactions without being altered themselves
- substances that enzymes convert into products in these reactions is called substrates – general equation for an enzyme-catalysed reaction is substrate (enzyme–>) product
- many different enzymes are needed because they only catalyse one biochemical reaction and thousands of reactions take place in cells nearly all of which need to be catalysed – enzyme-substrate specificity
- look at the mechanism by which enzymes speed up reactions – substrate, substrates binding to a special region on the surface of the enzyme called the active site
- shape and chemical properties of the active site and the substrate match each other = allows the substrate to bind not other substances
- substances are converted into products while they are bound to the active site and the products are then released, freeing the active site to catalyse another reaction
Outline the three stages of enzyme activity.
- Outline: Give a brief account or summary
Enzyme reactions typically occur in aqueous solutions. Consequently, the substrate and enzyme are usually moving randomly within the solution. This is referred to as Brownian motion. Sometimes an enzyme may be fixed in position (e.g. membrane-bound) – this serves to localize reactions to particular sites
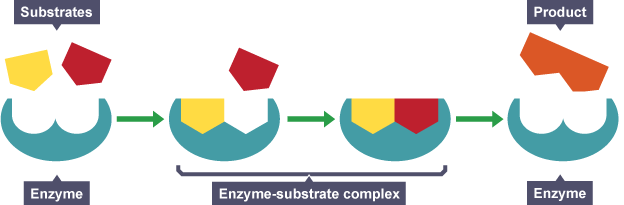
Four Steps of Enzyme Action
Four Steps of Enzyme Action
- The enzyme and the substrate are in the same area. Some situations have more than one substrate molecule that the enzyme will change.
- The enzyme grabs on to the substrate at a special area called the active site. The combination is called the enzyme/substrate complex. Enzymes are very, very specific and don't just grab on to any molecule. The active site is a specially shaped area of the enzyme that fits around the substrate. The active site is like the grasping claw of the robot on the assembly line. It can only pick up one or two parts.
- A process called catalysis happens. Catalysis is when the substrate is changed. It could be broken down or combined with another molecule to make something new. It will break or build chemical bonds. When done, you will have the enzyme/products complex.
- The enzyme releases the product. When the enzyme lets go, it returns to its original shape. It is then ready to work on another molecule of substrate.
Explain how temperature, pH and substrate concentration affect the rate of activity of enzymes.
- Explain: Give a detailed account including reasons or causes
The activity of an Enzyme is affected by its environmental conditions. Changing these alter the rate of reaction caused by the enzyme. In nature, organisms adjust the conditions of their enzymes to produce an Optimum rate of reaction, where necessary, or they may have enzymes which are adapted to function well in extreme conditions where they live.
Temperature
pH - Acidity and Basicity
pH measures the Acidity and Basicity of a solution. It is a measure of the Hydrogen Ion (H+) concentration, and therefore a good indicator of the Hydroxide Ion (OH-) concentration. It ranges from pH1 to pH14. Lower pH values mean higher H+ concentrations and lower OH- concentrations.
Concentration
Substrate Concentration
Increasing Substrate Concentration increases the rate of reaction. This is because more substrate molecules will be colliding with enzyme molecules, so more product will be formed.
Enzyme Concentration
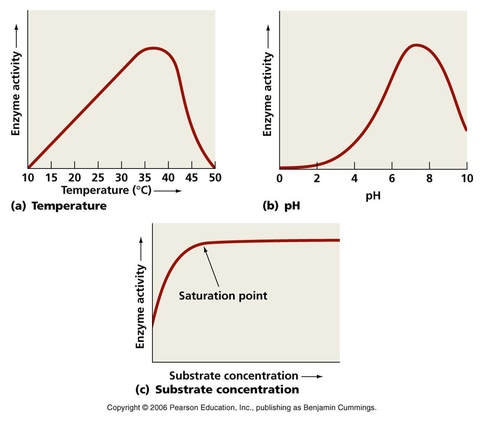
Temperature
- Low temperatures result in insufficient thermal energy for the activation of an enzyme-catalysed reaction to proceed
- Increasing the temperature will increase the speed and motion of both enzyme and substrate, resulting in higher enzyme activity
- This is because a higher kinetic energy will result in more frequent collisions between the enzymes and substrates
- At an optimal temperature (may vary for different enzymes), the rate of enzyme activity will be at its peak
- Higher temperatures will cause enzyme stability to decrease, as the thermal energy disrupts the enzyme’s hydrogen bonds
- This causes the enzyme (particularly the active site) to lose its shape, resulting in the loss of activity (denaturation)
pH - Acidity and Basicity
pH measures the Acidity and Basicity of a solution. It is a measure of the Hydrogen Ion (H+) concentration, and therefore a good indicator of the Hydroxide Ion (OH-) concentration. It ranges from pH1 to pH14. Lower pH values mean higher H+ concentrations and lower OH- concentrations.
- Changing the pH will alter the charge of the enzyme, which in turn will alter protein solubility and overall shape
- Changing the shape or charge of the active site will diminish its ability to bind the substrate, abrogating enzyme function
- Enzymes have an optimal pH (may differ between enzymes) and moving outside this range diminishes enzyme activity
- Small changes in pH above or below the Optimum do not cause a permanent change to the enzyme, since the bonds can be reformed. However, extreme changes in pH can cause enzymes to Denature and permanently lose their function.
Concentration
- Changing the Enzyme and Substrate concentrations affect the rate of reaction of an enzyme-catalysed reaction. Controlling these factors in a cell is one way that an organism regulates its enzyme activity and so its Metabolism.
- Changing the concentration of a substance only affects the rate of reaction if it is the limiting factor: that is, it the factor that is stopping a reaction from preceding at a higher rate.
Substrate Concentration
Increasing Substrate Concentration increases the rate of reaction. This is because more substrate molecules will be colliding with enzyme molecules, so more product will be formed.
- Increasing substrate concentration will increase the activity of a corresponding enzyme
- More substrates mean there is an increased chance of enzyme and substrate colliding and reacting within a given period
- After a certain point, the rate of activity will cease to rise regardless of any further increases in substrate levels
- This is because the environment is saturated with substrate and all enzymes are bound and reacting (Vmax)
Enzyme Concentration
- Increasing Enzyme Concentration will increase the rate of reaction, as more enzymes will be colliding with substrate molecules.
- However, this too will only have an effect up to a certain concentration, where the Enzyme Concentration is no longer the limiting factor..
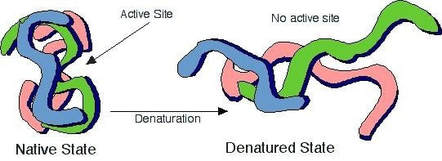
State the effect of denaturation on enzyme structure and function.
- State: Give a specific name, value or other brief answer without explanation or calculation.
The important part of an enzyme is called the active site. This is where specific molecules bind to the enzyme and the reaction occurs.
Anything that changes the shape of the active site stops the enzyme from working. This is similar to a key that opens a door lock. It does not matter what a key handle looks like, but if you change the shape of the ‘teeth’ the key no longer works.
The shape of the active site is affected by pH. This is why enzymes will only work at a specific pH, as well as a specific temperature. Change the pH and the enzyme stops working.
Increasing the temperature to 60°C will cause a permanent change to the shape of the active site. This is why enzymes stop working when they are heated. We say they have become denatured.
Anything that changes the shape of the active site stops the enzyme from working. This is similar to a key that opens a door lock. It does not matter what a key handle looks like, but if you change the shape of the ‘teeth’ the key no longer works.
The shape of the active site is affected by pH. This is why enzymes will only work at a specific pH, as well as a specific temperature. Change the pH and the enzyme stops working.
Increasing the temperature to 60°C will cause a permanent change to the shape of the active site. This is why enzymes stop working when they are heated. We say they have become denatured.
Explain how immobilized enzymes are widely used in industry.
- Explain: Give a detailed account including reasons or causes
Enzyme immobilization is the process of confining the enzyme molecule to a distinct phase from the one where in the substrates and the products are present. This allows the enzyme to retain its catalytic activity and be repeatedly and continuously used.
Applications of enzyme immobilization
Applications of enzyme immobilization
- Industrial production – industrial production of antibiotics, beverages, amino acids etc. uses immobile enzymes or who cells
- Biomedical applications – immobilized enzymes are widely used in the diagnosis and treatment of many diseases. Immobilized enzymes can be used to overcome inborn metabolic disorders by the supply of immobilized enzymes. Mobilization techniques are effectively used in drug delivery systems especially to oncogenic sites
- Food industry – enzymes like pectinases and cellulases immobilized on suitable carriers are successfully used in the production of jams, jellies and syrups from fruits and vegetables
- Research – a research activity extensively uses many enzymes. The use of immobilized enzyme allows researchers to increase the efficiency of different enzymes such as Horse Radish Peroxidase (HRP) in blotting experiments and different Proteases for cell or organelle lysis
- Production of biodiesel from vegetable oils
- Wastewater management – treatment of sewage and industrial effluents.
- Textile industry – scouring, bio-[polishing and desizing of fabrics
- Detergent industry – immobilization of lipase enzyme for effective dirt removal from clothes
Outline four reasons for using lactase in food processing.
- Outline: Give a brief account or summary

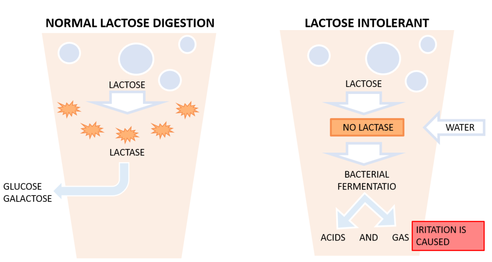
Lactose is the sugar found in milk. It can be broken down by the enzyme lactase into glucose and galactose. However some people lack this enzyme and so cannot break down lactose leading to lactose intolerance. Lactose intolerant people need to drink milk that has been lactose reduced. Lactose-free milk can be made in two ways. The first involves adding the enzyme lactase to the milk so that the milk contains the enzyme. The second way involves immobilizing the enzyme on a surface or in beads of a porous material. The milk is then allowed to flow past the beads or surface with the immobilized lactase. This method avoids having lactase in the milk.
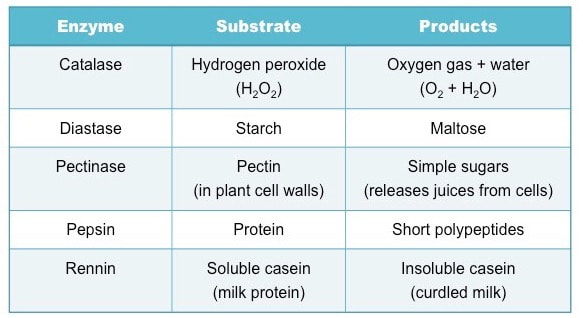
Identify the different types of enzymes, their substrates and products
- Identify: Provide an answer from a number of possibilities. Recognize and state briefly a distinguishing fact or feature.
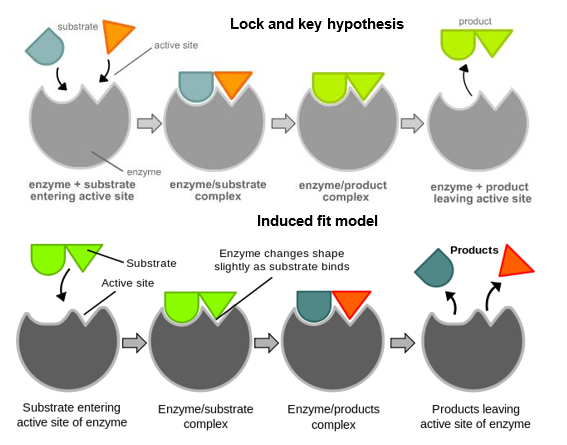
Outline the two models used to describe the way enzymes interact with substrates.
- Outline: Give a brief account or summary.
The two models to explain the actions of enzymes with substrates are the Lock and Key model & Induced fit model.
In lock and key the enzyme is the lock and the substrate is the key. As with a lock and the key that opens it the shapes must be complementary and this shape can not change.
Induced fit looks at the active site of enzymes as being slightly more flexible and initially uncomplementary. It suggests that it is the binding of the substrate to enzyme that causes the active site to change into a complementary shape and allow the enzyme-substrate complex to form.
In lock and key the enzyme is the lock and the substrate is the key. As with a lock and the key that opens it the shapes must be complementary and this shape can not change.
Induced fit looks at the active site of enzymes as being slightly more flexible and initially uncomplementary. It suggests that it is the binding of the substrate to enzyme that causes the active site to change into a complementary shape and allow the enzyme-substrate complex to form.
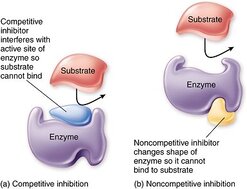
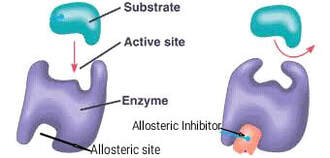
Distinguish between competitive and noncompetitive inhibitors.
- Distinguish: Make clear the differences between two or more concepts or items.
An enzyme inhibitor is a molecule that disrupts the normal reaction pathway between an enzyme and a substrate. Enzyme inhibitors can be either competitive or non-competitive depending on their mechanism of action
- Competitive inhibition - the inhibitor binds directly to the active site of the enzyme. This prevents the substrate from binding to the enzyme and forming the enzyme-substrate complex. It is directly competing with the substrate.
- Uncompetitive inhibition - the inhibitor binds only to the substrate-enzyme complex.
- Non-competitive inhibition - the inhibitor binds to a site on the enzyme that is NOT the active site. In doing so, it alters the conformation of the active site, meaning that the substrate can no longer bind to the active site on the enzyme.
- Allosteric regulation - an allosteric inhibitor by binding to allosteric site alters the protein conformation in active site of enzyme which consequently changes the shape of active site. Thus enzyme no longer remains able to bind to its specific substrate. Hence enzyme is unable to perform it's catalytic activity i.e enzyme is now inactive.
Key Term:
|
enzyme
globular shape catalyst product allosteric |
active site
amino acid denature inhibitor |
substrate
covalent bond immobilized competitive inhibition |
-denaturation
lactose lock and key non-competitive inhibition |
lactase
enzyme specificity induced fit |
Class Material:
Correct use of terminology is a key skill in Biology. It is essential to use key terms correctly when communicating your understanding, particularly in assessments. Use the quizlet flashcards or other tools such as learn, scatter, space race, speller and test to help you master the vocabulary
Useful Links:
KScience
Lew Ports Biology Page
Chemistry for Kids
Enzyme Lab
Clear and simple animations, with factors from KScience.co.uk
More simple animations from John Gianni
How enzymes work from McGraw Hill
What is an enzyme? from Northland (the best one – including inhibitors, pathways and feedback inhibition)
A full collection of savable enzyme animations from Husam Medical
Enzyme action and the hydrolysis of sucrose from McGraw Hill
Protein denaturation from McGraw Hill
The pH scale PhET Lab (allow Java to run)
News:
Cancer Cell Enzymes BBC News Health
New Small Molecule Catalyst Phys.org
Video Clips:
Paul Andersen explains how enzymes are used to break down substrates. The correct shape of the active site allows a key/lock fit between the enzyme and the substrate. The enzyme catalase is used to break down hydrogen peroxide. The importance of cofactors and coenzymes is emphasized. Competitive and allosteric inhibition is also included.
The Amoeba Sisters explain enzymes and how they interact with their substrates
Enzymes are really important proteins, that speed up the rates of reactions such as in photosynthesis, respiration and protein synthesis.
The enzymes and substrates are always moving, and occasionally they collide at the right speed and orientation so that the substrate fits into the enzyme at the active site
The enzymes and substrates are always moving, and occasionally they collide at the right speed and orientation so that the substrate fits into the enzyme at the active site
What are enzymes? Why they're nature's little factory workers. They chop up certain things! They build up others! Pretty amazing the kind of chemistry nature can do given enormous polypeptide chains with unfathomable variability and billions of years of evolution, no?
Factors that Affect Enzymes
Dr. Kiki breaks down the breakdown of proteins.
All adult mammals but humans are lactose intolerant. Follow human geneticist Spencer Wells, director of the Genographic Project of the National Geographic Society, as he tracks down the genetic and societal changes associated with the ability to digest lactose as adults—or lactose tolerance
Allosteric Regulation of Enzymes