topic 3: Macromolecules
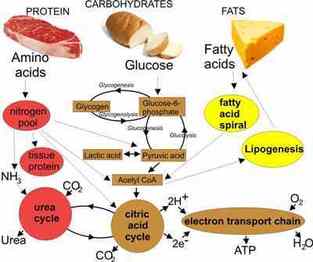
Like all matter, life is built from atoms — the basic units of matter that link together to form molecules. Macromolecules are the molecules of life and are built around chains of carbon atoms that are often quite long.
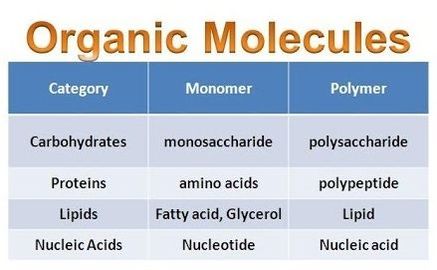
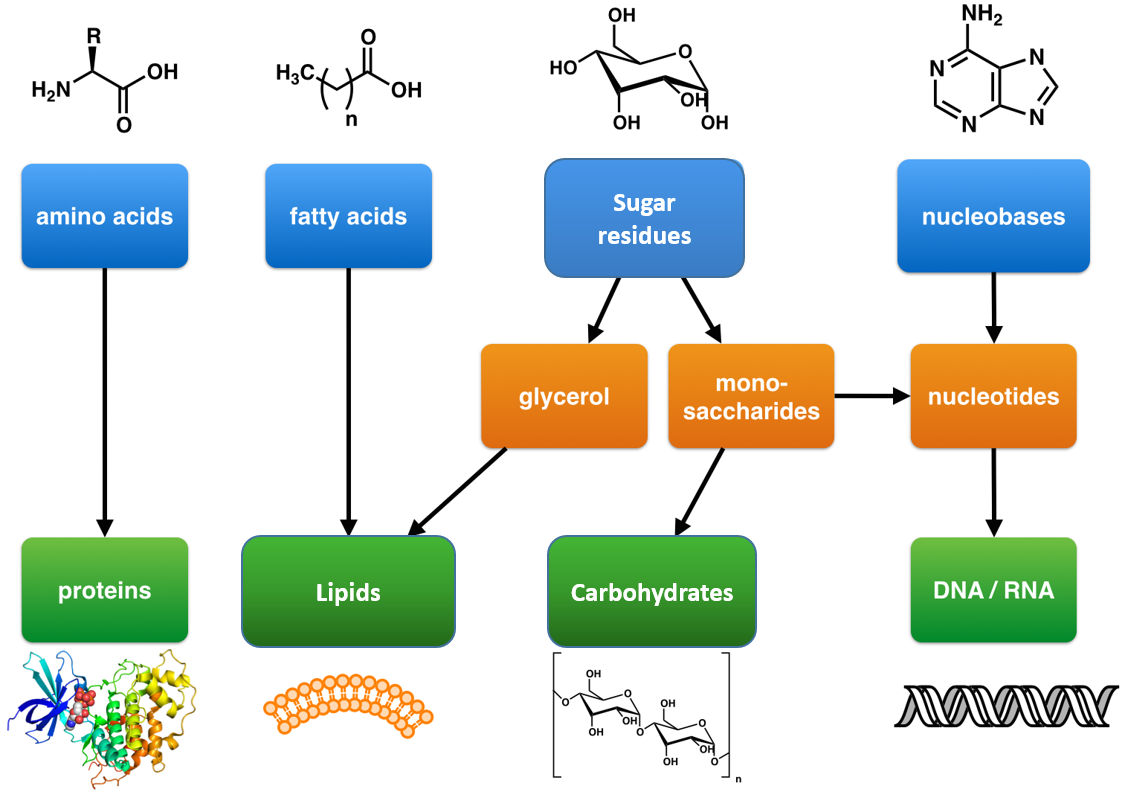
Proteins, carbohydrates, nucleic acids, and lipids are the four major classes of biological macromolecules—large molecules necessary for life that are built from smaller organic molecules. Macromolecules are made up of single units known as monomers that are joined by covalent bonds to form larger polymers.
Proteins, carbohydrates, nucleic acids, and lipids are the four major classes of biological macromolecules—large molecules necessary for life that are built from smaller organic molecules. Macromolecules are made up of single units known as monomers that are joined by covalent bonds to form larger polymers.
Explain why carbon’s structure allows it form molecules essential for life.
- Explain: Give a detailed account including reasons or causes.
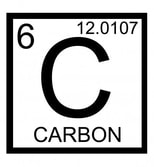
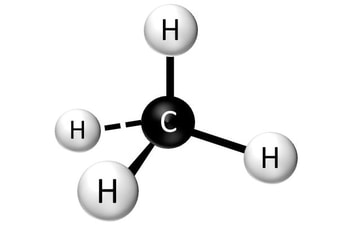
Carbon is the most important element to life. Without this element, life as we know it would not exist. As you will see, carbon is the central element in compounds necessary for life-organic compounds. Carbon has an exceptional ability to bind with a wide variety of other elements. Carbon makes four electrons available to form covalent chemical bonds, allowing carbon atoms to form multiple stable bonds with other small atoms, including hydrogen, oxygen, and nitrogen. Carbon atoms can also form stable bonds with other carbon atoms.
Carbon has the ability to form very long chains of interconnecting C-C bonds. This property allows carbon to form the backbone of organic compounds, carbon-containing compounds, which are the basis of all known organic life.
The properties of carbon make it the backbone of the organic molecules which form living matter.
- Carbon is a such a versatile element because it can form four covalent bonds.
- Carbon skeletons can vary in length, branching, and ring structure.
- The functional groups of organic molecules are the parts involved in chemical reactions.
- Organic molecules important for life include relatively small monomers as well as large polym
Identify the monomer and polymer structures of organic molecules
- Identify: Provide an answer from a number of possibilities. Recognize and state briefly a distinguishing fact or feature.
A monomer is a type of molecule that has the ability to chemically bond with other molecules in a long chain; a polymer is a chain of an unspecified number of monomers. Essentially, monomers are the building blocks of polymers, which are more complex type of molecules.
Identify the chemical elements present in carbohydrates, proteins and lipids (fats and oils)
- Identify: Provide an answer from a number of possibilities. Recognize and state briefly a distinguishing fact or feature.
Carbohydrate:
- The elements present in a carbohydrate is Carbon, Hydrogen and Oxygen (CHO). The simple molecule in carbohydrates are sugars and the larger molecules are the polysaccharites e.g. starch.
- The elements present in a protein is Carbon, Hydrogen, Oxygen and Nitrogen (CHON). The simple molecules of proteins are amino acids and the larger molecules are proteins itself.
- The elements present in lipids are Carbon, Hydrogen, Oxygen (CHO). Lipids can be split into two categories - the fats and the oils.
Outline the role of condensation and hydrolysis in cells
- Outline: Give a brief account or summary.
Condensation is a chemical process by which 2 molecules are joined together to make a larger, more complex, molecule, with the loss of water. It is the basis for the synthesis of all the important biological macromolecules (carbohydrates, proteins, lipids, nucleic acids) from their simpler sub-units.
Hydrolysis is the opposite to condensation. A large molecule is split into smaller sections by breaking a bond, adding -H to one section and -OH to the other. The products are simpler substances. Since it involves the addition of water, this explains why it is called hydrolysis, meaning splitting by water.
Hydrolysis is the opposite to condensation. A large molecule is split into smaller sections by breaking a bond, adding -H to one section and -OH to the other. The products are simpler substances. Since it involves the addition of water, this explains why it is called hydrolysis, meaning splitting by water.
Summarize the roles carbohydrates play in biological systems.
- Summarize: Abstract a general theme or major point(s).
Carbohydrates are also known as sugars. They are one of the most abundant biomolecule. More than 50% of the carbon in organic compounds is found in carbohydrates
The primary role of carbohydrates is to supply energy to all cells in the body. Many cells prefer glucose as a source of energy versus other compounds like fatty acids
Sources
The primary role of carbohydrates is to supply energy to all cells in the body. Many cells prefer glucose as a source of energy versus other compounds like fatty acids
- Key intermediates of metabolism (sugars)
- Structural components of plants (cellulose)
- Central to materials of industrial products: paper, lumber, fibers
- Key component of food sources: sugars, flour, vegetable fiber
Sources
- Glucose is produced in plants through photosynthesis from CO2 and H2O
- Glucose is converted in plants to other small sugars and polymers (cellulose, starch)
- Dietary carbohydrates provide the major source of energy required by organisms
Identify the structure of carbohydrates
Identify: Provide an answer from a number of possibilities. Recognize and state briefly a distinguishing fact or feature.
Identify: Provide an answer from a number of possibilities. Recognize and state briefly a distinguishing fact or feature.
Carbohydrates are biological molecules made of carbon, hydrogen, and oxygen in a ratio of roughly one carbon atom to one water molecule. This composition gives carbohydrates their name: they are made up of carbon (carbo-) plus water (-hydrate).
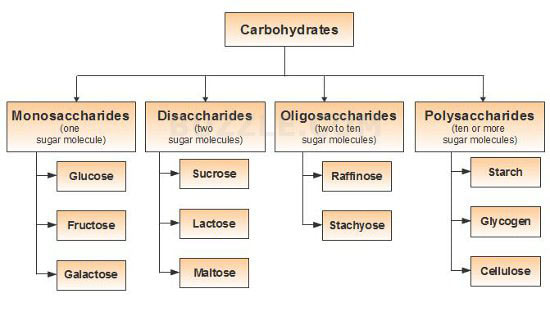
Carbohydrate chains come in different lengths, and biologically important carbohydrates belong to three categories:
Carbohydrate chains come in different lengths, and biologically important carbohydrates belong to three categories:
- monosaccharides
- disaccharides,
- polysaccharides.
Distinguish between monosaccharides, disaccharides, and polysaccharides.
- Distinguish: Make clear the differences between two or more concepts or items.
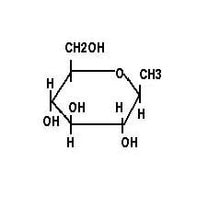
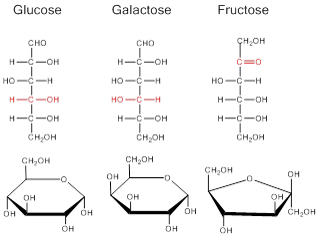
Monosaccharide carbohydrates are those carbohydrates that cannot be hydrolyzed further to give simpler units. The word monosaccharide is derived from mono, meaning "one", and saccharide, meaning "sugar". The common monosaccharides are glucose, fructose, and galactose. Each simple sugar has a cyclic structure and is composed of carbon, hydrogen and oxygen in ratios of 1:2:1
- Glucose is the main sugar metabolized by the body for energy
- Galactose is nearly identical to glucose in structure. Galactose is not normally found in nature in large quantities, however it combines with glucose to form lactose in milk
- Fructose, or fruit sugar, is a simple ketonic monosaccharide found in many plants, where it is often bonded to glucose to form the disaccharide sucrose
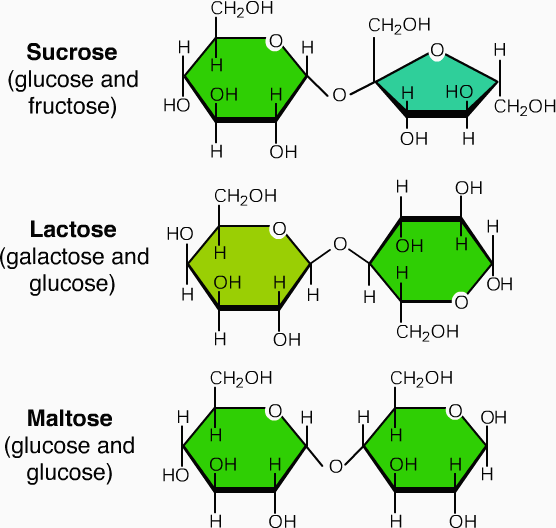
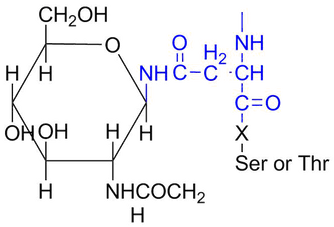
Disaccharides, meaning "two sugars", are commonly found in nature as sucrose, lactose and maltose. They are formed by a condensation reaction where one molecule of water condenses or is released during the joining of two monosaccharides. The type of bond that is formed between the two sugars is called a glycosidic bond.
- Lactose is a disaccharide formed through the condensation of glucose and galactose. It is found naturally in milk.
- Sucrose is found in common table sugar and is composed of glucose and fructose linked.
- Maltose consists of two glucose molecules joined. Maltose, or malt sugar, is a disaccharide formed by a dehydration reaction between two glucose molecules. Maltose is used in alcohol production. Through a process called fermentation, glucose, maltose and other sugars are converted to ethanol by yeast cells in the absence of oxygen. Although maltose is uncommon in nature, it can be formed through the breakdown of starch by the enzymes of the mouth
Oligosaccharides - These are complex carbohydrates that consist of three to ten sugars. They are rich in vitamins and minerals; and, because they are fiber-rich, they are slower to digest than a simple carbohydrate. Oligosaccharides can have many functions including cell recognition and cell binding
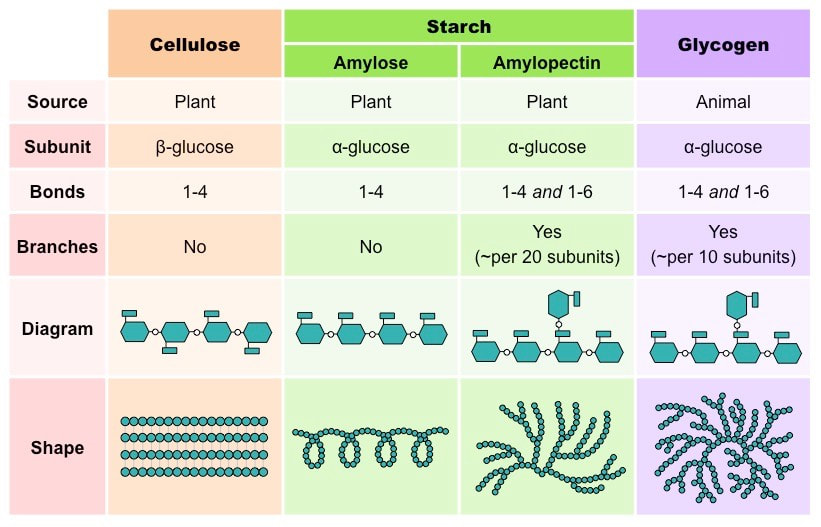
Polysaccharides are usually monomers and consist of thousands of repeating glucose units. Naturally, they allow for the storage of large quantities of glucose.
- Starch is the stored form of sugars in plants and is made up of a mixture of amylose and amylopectin (both polymers of glucose). Plants are able to synthesize glucose, and the excess glucose, beyond the plant’s immediate energy needs, is stored as starch in different plant parts, including roots and seeds. The starch in the seeds provides food for the embryo as it germinates and can also act as a source of food for humans and animals.
- Glycogen is amylopectin with very short distances between the branching side-chains. Starch from plants is hydrolyzed in the body to produce glucose. Glucose passes into the cell and is used in metabolism. Inside the cell, glucose can be polymerized to make glycogen which acts as a carbohydrate energy store
- Cellulose is the most abundant natural biopolymer. The cell wall of plants is mostly made of cellulose; this provides structural support to the cell. Wood and paper are mostly cellulosic in nature
- Chitin is a cellulose-like polymer existing in the hard exoskeleton of insects, crustaceans. Chitin is also a major component of fungal cell walls
Summarize the roles lipids play in biological systems.
- Summarize: Abstract a general theme or major point(s).
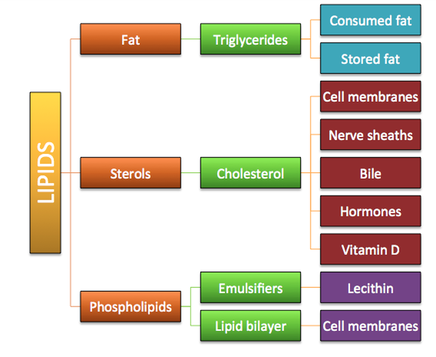
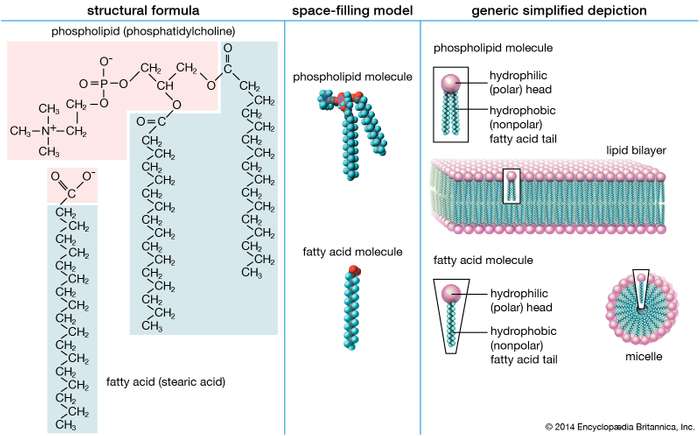
Lipids are composed of carbon, hydrogen and oxygen atoms, and in some cases contain phosphorus, nitrogen, sulfur and other element. A lipid molecule consists of two kinds of parts: a glycerol backbone and three fatty acid tails. Glycerol is a small organic molecule with three hydroxyl (OH) groups, while a fatty acid consists of a long hydrocarbon chain attached to a carboxyl group.
Lipids are also known as a triglyceride. It is made up of a molecule known as glycerol that is connected to one, two, or three fatty acids. Glycerol is the basis of all fats and is made up of a three-carbon chain that connects the fatty acids together. A fatty acid is just a long chain of carbon atoms connected to each other.
Lipids can serve a diverse range of functions within a cell, including:
Lipids are also known as a triglyceride. It is made up of a molecule known as glycerol that is connected to one, two, or three fatty acids. Glycerol is the basis of all fats and is made up of a three-carbon chain that connects the fatty acids together. A fatty acid is just a long chain of carbon atoms connected to each other.
Lipids can serve a diverse range of functions within a cell, including:
- Storage of energy for long-term use (e.g. triglycerides)
- Hormonal roles (e.g. steroids such as estrogen and testosterone)
- Insulation – both thermal (triglycerides) and electrical (sphingolipids)
- Protection of internal organs (e.g. triglycerides and waxes)
- Structural components of cells (e.g. phospholipids and cholesterol)
Describe the structure and function of lipids
- Describe: Give a detailed account or picture of a situation, event, pattern or process.
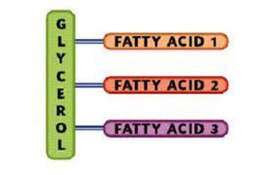
The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries as well as in nanotechnology. Lipids are made of a triglyceride that is made from the alcohol glycerol, plus fatty acidsLipids tend to be hydrophobic, nonpolar, and made up mostly of hydrocarbon chains. Some lipids are amphipathic—part of their structure is hydrophilic and another part, usually a larger section, is hydrophobic. Amphipathic lipids exhibit a unique behavior in water: they spontaneously form ordered molecular aggregates, with their hydrophilic ends on the outside, in contact with the water, and their hydrophobic parts on the inside, shielded from the water. This property is key to their role as the fundamental components of cellular and organelle membranes.
Types of Lipids
Types of Lipids
- Triglycerides: Function as a long-term energy source in animals (fats) and plants (oils)
- Phospholipids: Structural component of cell membranes
- Steroids: Act as hormones in plants and animals, and is a structural component of animal cell membranes (cholesterol)
- Waxes: Act as a protective layer against water loss in plant leaves and animal skin
- Carotenoids: Light-absorbing accessory pigment in plants (involved in photosynthesis)
- Glycolipids: Complexes of carbohydrate and lipid that function as cell receptor and cell recognition molecules
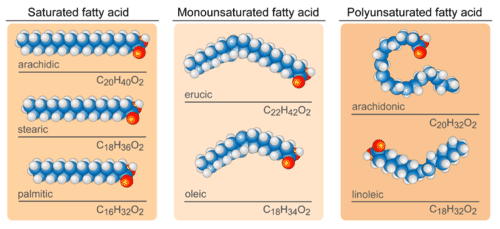
Distinguish between saturated and unsaturated fats.
- Distinguish: Make clear the differences between two or more concepts or items.
In a fatty acid chain,
Origin
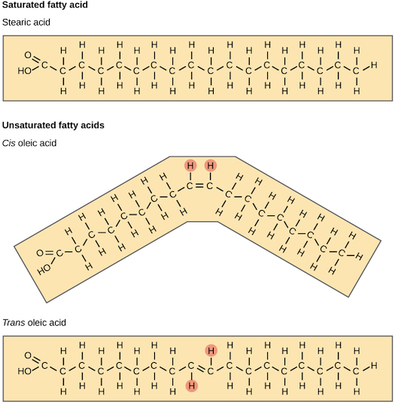
Unsaturated fats or oils are usually of plant origin and contain cis unsaturated fatty acids. Cis and trans indicate the configuration of the molecule around the double bond. If hydrogens are present in the same plane, it is referred to as a cis fat; if the hydrogen atoms are on two different planes, it is referred to as a trans fat. The cis double bond causes a bend or a “kink” that prevents the fatty acids from packing tightly, keeping them liquid at room temperature
- Saturated - single bonds between neighboring carbons in the hydrocarbon chain. Saturated fatty acids are saturated with hydrogen since single bonds increase the number of hydrogens on each carbon. Stearic acid and palmitic acid, which are commonly found in meat, are examples of saturated fats.
- Unsaturated - contains a double bond. Oleic acid is an example of an unsaturated fatty acid. If there is only one double bond in the molecule, then it is known as a monounsaturated fat; e.g. olive oil. If there is more than one double bond, then it is known as a polyunsaturated fat; e.g. canola oil. Unsaturated fats help to lower blood cholesterol levels whereas saturated fats contribute to plaque formation in the arteries.
- Unsaturated fats - most are liquid at room temperature and are called oils.
- Saturated fats - most are solid at room temperature and are called fats
Origin
- Unsaturated fats - usually found in plants
- Saturated fats - usually found in animals
Unsaturated fats or oils are usually of plant origin and contain cis unsaturated fatty acids. Cis and trans indicate the configuration of the molecule around the double bond. If hydrogens are present in the same plane, it is referred to as a cis fat; if the hydrogen atoms are on two different planes, it is referred to as a trans fat. The cis double bond causes a bend or a “kink” that prevents the fatty acids from packing tightly, keeping them liquid at room temperature
Trans fats occur naturally, in small quantities, in meat and dairy products from ruminants.
Most trans fats consumed today, however, are industrially created as a side effect of partial hydrogenation of plant oils.
Partial hydrogenation changes a fat's molecular structure (raising its melting point and reducing rancidity) but this process also results in a proportion of the changed fat becoming trans fat.
Unlike other fats, trans fats are neither required nor beneficial for health.
Eating trans fat increases the risk of coronary heart disease.
Most trans fats consumed today, however, are industrially created as a side effect of partial hydrogenation of plant oils.
Partial hydrogenation changes a fat's molecular structure (raising its melting point and reducing rancidity) but this process also results in a proportion of the changed fat becoming trans fat.
Unlike other fats, trans fats are neither required nor beneficial for health.
Eating trans fat increases the risk of coronary heart disease.
Summarize the roles proteins play in biological systems.
- Summarize: Abstract a general theme or major point(s).
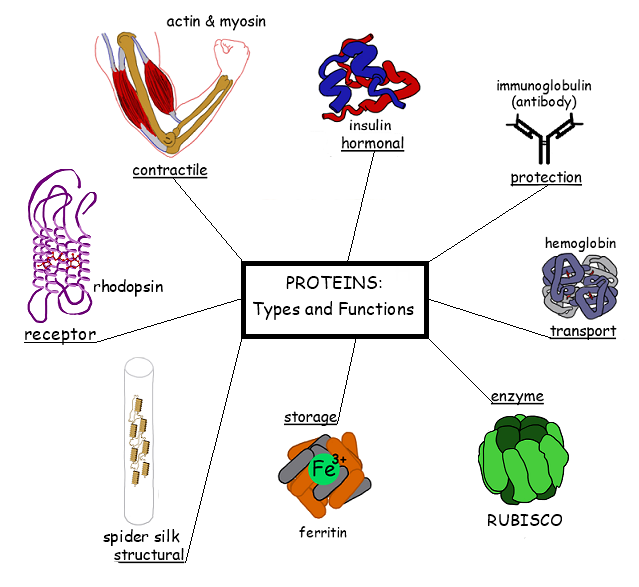
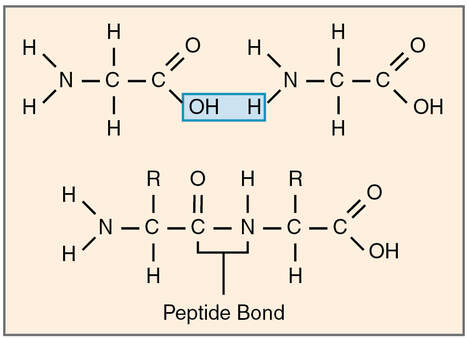
Proteins are a class of macromolecules that perform a diverse range of functions for the cell. They help in metabolism by providing structural support and by acting as enzymes, carriers, or hormones. The building blocks of proteins (monomers) are amino acids. Each amino acid has a central carbon that is linked to an amino group, a carboxyl group, a hydrogen atom, and an R group or side chain. There are 20 commonly occurring amino acids, each of which differs in the R group. Each amino acid is linked to its neighbors by a peptide bond. A long chain of amino acids is known as a polypeptide.
- Antibodies - bind to specific foreign particles, such as viruses and bacteria, to help protect the body. Example immunoglobulin
- Enzymes - carry out almost all of the thousands of chemical reactions that take place in cells. They also assist with the formation of new molecules by reading the genetic information stored in DNA. Example Amylase
- Messenger proteins - hormones, transmit signals to coordinate biological processes between different cells, tissues, and organs. Example - Growth hormone
- Structural component - Provide structure and support for cells. On a larger scale, they also allow the body to move. Example Actin
- Transport/storage - Bind and carry atoms and small molecules within cells and throughout the body. Example Ferritin
Describe the structure and function proteins.
- Describe: Give a detailed account or picture of a situation, event, pattern or process.
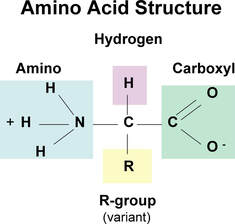
Amino acids play central roles both as building blocks of proteins and as intermediates in metabolism. The 20 amino acids that are found within proteins convey a vast array of chemical versatility. The precise amino acid content, and the sequence of those amino acids, of a specific protein, is determined by the sequence of the bases in the gene that encodes that protein
Proteins are biological polymers composed of amino acids. Amino acids, linked together by peptide bonds, form a polypeptide chain. One or more polypeptide chains twisted into a 3-D shape. Proteins have complex shapes that include various folds, loops, and curves. Folding in proteins happens spontaneously. Chemical bonding between portions of the polypeptide chain aid in holding the protein together and giving it its shape.
The common property of all proteins is that they consist of long chains of α-amino (alpha amino) acids. The general structure of α-amino acids is shown in . The α-amino acids are so called because the α-carbon atom in the molecule carries an amino group (―NH2); the α-carbon atom also carries a carboxyl group (―COOH). The amino acids present in proteins differ from each other in the structure of their side (R) chains.
Structures of Proteins
Proteins are biological polymers composed of amino acids. Amino acids, linked together by peptide bonds, form a polypeptide chain. One or more polypeptide chains twisted into a 3-D shape. Proteins have complex shapes that include various folds, loops, and curves. Folding in proteins happens spontaneously. Chemical bonding between portions of the polypeptide chain aid in holding the protein together and giving it its shape.
The common property of all proteins is that they consist of long chains of α-amino (alpha amino) acids. The general structure of α-amino acids is shown in . The α-amino acids are so called because the α-carbon atom in the molecule carries an amino group (―NH2); the α-carbon atom also carries a carboxyl group (―COOH). The amino acids present in proteins differ from each other in the structure of their side (R) chains.
Structures of Proteins
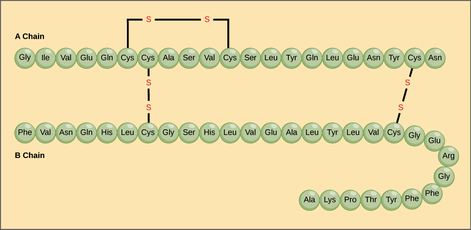
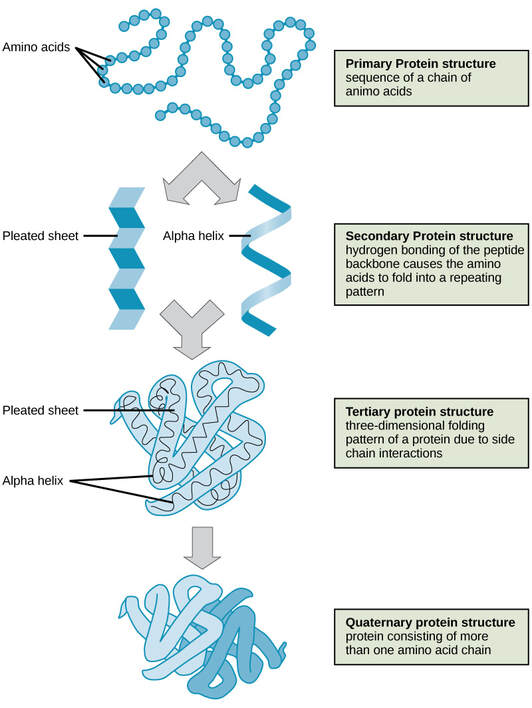
- Primary — simplest level of protein structure. As proteins are being built, they begin as a straight chain of amino acids. Sometimes chains can bond to each other with two sulfur (S) atoms. Those bonds would be called a disulfide bridge.
- Example - insulin
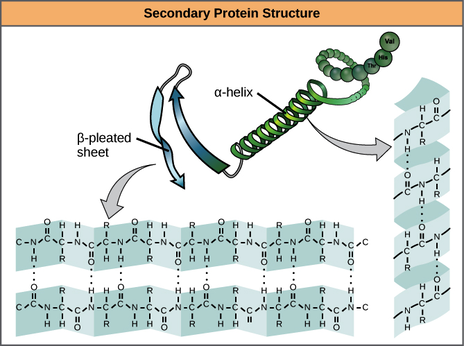
Secondary - Local folded structures that form within a polypeptide due to interactions between atoms of the backbone In the amino acid chain. Each of the amino acids interacts with the others and it twists like a corkscrew (alpha helix) or it takes the shape of a folded sheet (beta sheet). This structure resembles a coiled spring and is secured by hydrogen bonding in the polypeptide chain. 2-dimensional structures formed due to hydrogen binding between hydrogen of amine groups and oxygen of the carbonyl groups
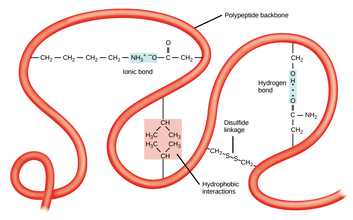
Tertiary structure - involves the three-dimensional folding of a protein due to interactions of amino acid. Tertiary structure is a result of interactions between the R groups of the amino acids that make up the protein.
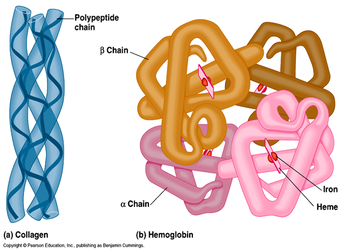
- Quaternary - Many proteins are made up of a single polypeptide chain and have only three levels of structure (the ones we’ve just discussed). However, some proteins are made up of multiple polypeptide chains, also known as subunits
- Example - hemoglobin.
Explain how a peptide bond forms between two amino acids.
- Explain: Give a detailed account including reasons or causes.
The sequence and the number of amino acids ultimately determine the protein’s shape, size, and function. Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. When two amino acids are covalently attached by a peptide bond, the carboxyl group of one amino acid and the amino group of the incoming amino acid combine and release a molecule of water. Any reaction that combines two monomers in a reaction that generates H2O as one of the products is known as a dehydration reaction, so peptide bond formation is an example of a dehydration reaction. The resulting chain of amino acids is called a polypeptide chain
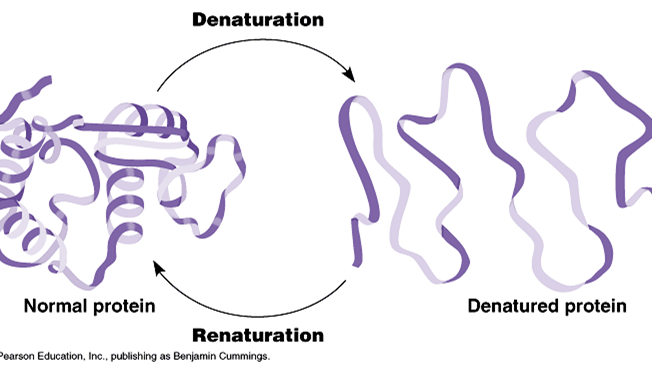
List three conditions under which proteins may be denatured.
- List: Give a sequence of brief answers with no explanation.
The shape of a protein determines its function, and they are fine tuned to homeostasis conditions. Anything that upsets these conditions will denature the protein.The interactions, such as hydrogen bonding , that dictate the tertiary structure of proteins are not as strong as covalent chemical bonds. Because these interactions are rather weak, they can be disrupted with relatively modest stresses. Denature of proteins involves the disruption and possible destruction of both the secondary and tertiary structures. Since denaturation reactions are not strong enough to break the peptide bonds, the primary structure (sequence of amino acids) remains the same after a denaturation process. Denaturation disrupts the normal alpha-helix and beta sheets in a protein and uncoils it into a random shape.
Temperature
pH
Salt
Temperature
- High levels of thermal energy may disrupt the hydrogen bonds that hold the protein together As these bonds are broken, the protein will begin to unfold and lose its capacity to function as intended. Temperatures at which proteins denature may vary, but most human proteins function optimally at body temperature (~37ºC)
pH
- Amino acids are zwitterions, neutral molecules possessing both negatively (COO–) and positively (NH3+) charged regions. Changing the pH will alter the charge of the protein, which in turn will alter protein solubility and overall shape. All proteins have an optimal pH which is dependent on the environment in which it functions (e.g. stomach proteins require an acidic environment to operate, whereas blood proteins function best at a neutral pH)
Salt
- These effects depend on several factors including the identity of the salt. Some salts, such as ammonium sulfate, tend to stabilize protein structures and increase the melting temperature. Others, such as calcium chloride, destabilize proteins and lower the melting temperature
Summarize the roles nucleotides play in biological systems.
- Summarize: Abstract a general theme or major point(s).
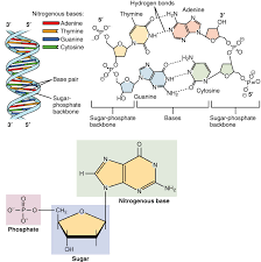
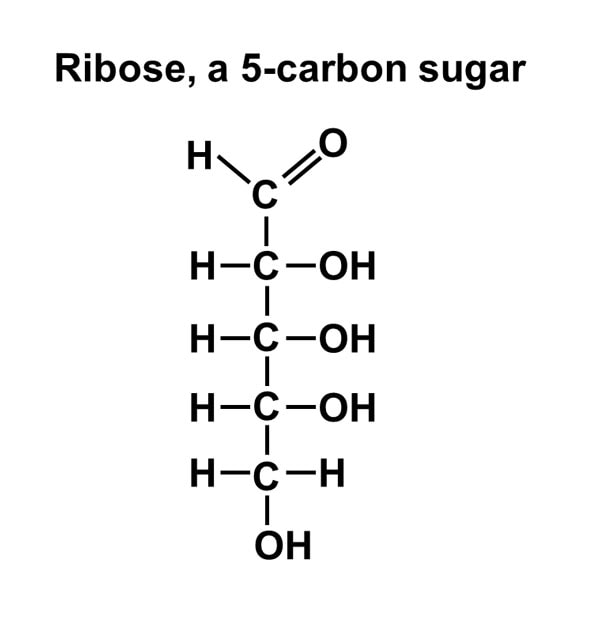
Nucleotides are monomers of nucleic acids, Nucleotides consist of a nitrogen-containing base, a five-carbon sugar and one or more phosphate groups. Cells contain many types of nucleotides, which are in constant flux between free and polymeric states. Nucleotides play central roles in many cellular processes, including metabolic regulation and the storage and utilization of genetic information.
Describe the structure and function of a nucleotide
- Describe: Give a detailed account or picture of a situation, event, pattern or process.
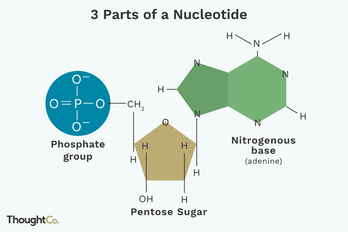
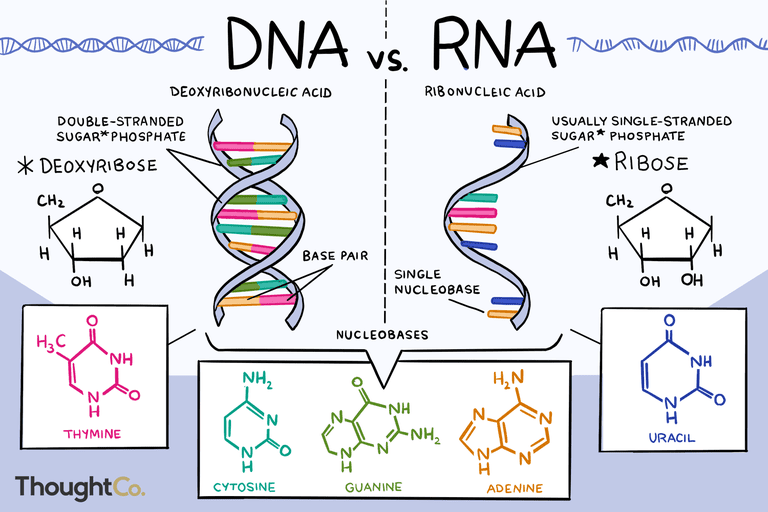
A nucleotide is an organic molecule that is the building block of DNA and RNA. They also have functions related to cell signaling, metabolism, and enzyme reactions. A nucleotide is made up of three parts: a phosphate group, a 5-carbon sugar, and a nitrogenous base. The four nitrogenous bases in DNA are adenine, cytosine, guanine, and thymine. RNA contains uracil, instead of thymine. A nucleotide within a chain makes up the genetic material of all known living things. They also serve a number of function outside of genetic information storage, as messengers and energy moving molecules
Nucleotide structure is simple, but the structure they can form together is complex. Each nucleotide within has a specific structure which enables this formation.
Nucleotide structure is simple, but the structure they can form together is complex. Each nucleotide within has a specific structure which enables this formation.
Describe how these monomers are linked to form a nucleic acid. Name the type of bond that holds two nucleotides together.
- Describe: Give a detailed account or picture of a situation, event, pattern or process.
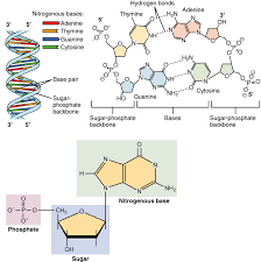 https://dlc.dcccd.edu/biology1-3/nucleic-acid
https://dlc.dcccd.edu/biology1-3/nucleic-acid
Nucleic acids are formed by repeated dehydration synthesis reactions between nucleotides.
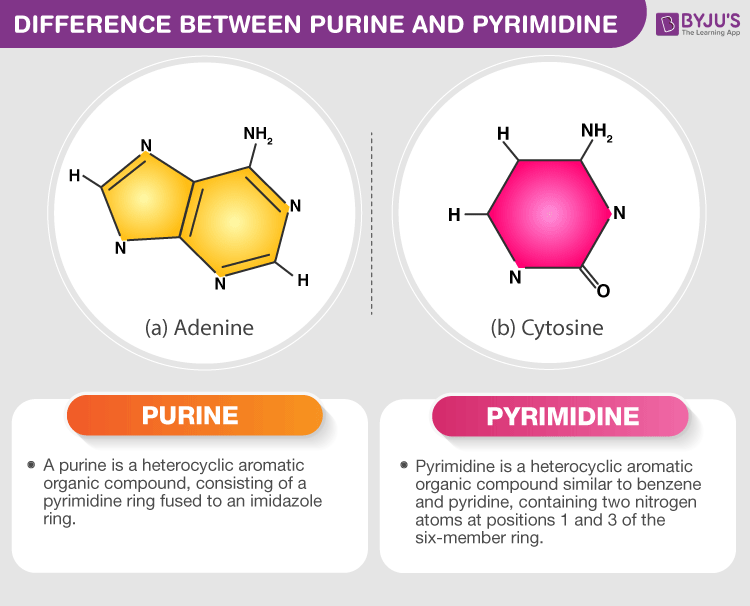
Distinguish between pyrimidine and purine
- Distinguish: Make clear the differences between two or more concepts or items.
Purines and pyrimidines are the two families of nitrogenous bases that make up nucleic acids
Each DNA strand has a ‘backbone’ that is made up of a sugar-phosphate chain. Attached to each one of these sugars is a nitrogenous base that is composed of carbon and nitrogen rings. The number of rings this base has determines whether the base is a purine (two rings) or a pyrimidine (one ring). The purines on one strand of DNA form hydrogen bonds with the corresponding pyrimidines on the opposite strand of DNA, and vice versa, to hold the two strands together. Within DNA molecules, this is their most important function and is known as base pairing. Because hydrogen bonds are not as strong as covalent bonds, base pairings can easily be separated, allowing for replication and transcription
Each DNA strand has a ‘backbone’ that is made up of a sugar-phosphate chain. Attached to each one of these sugars is a nitrogenous base that is composed of carbon and nitrogen rings. The number of rings this base has determines whether the base is a purine (two rings) or a pyrimidine (one ring). The purines on one strand of DNA form hydrogen bonds with the corresponding pyrimidines on the opposite strand of DNA, and vice versa, to hold the two strands together. Within DNA molecules, this is their most important function and is known as base pairing. Because hydrogen bonds are not as strong as covalent bonds, base pairings can easily be separated, allowing for replication and transcription
Compare and contrast DNA and RNA
- Compare and Contrast: Give an account of the similarities and differences between two (or more) items or situations, referring to both (all) of them throughout
DNA and RNA are made up of phosphates, sugar and nitrogen
- Found in the nucleus and cytoplasm of living organism
- Responsible for carrying genetic information and making proteins.
Describe the tests for glucose, starch, lipids and protein
- Describe: Give a detailed account or picture of a situation, event, pattern or process.
Test for glucose
Benedict's test:
Benedict's solution-blue solution containing copper (II) sulphate.
Reducing sugars such as glucose, maltose, fructose and lactose can reduce the copper (II) in Benedict's solution to copper (I).
Test for starch
Iodine test:
Benedict's test:
Benedict's solution-blue solution containing copper (II) sulphate.
Reducing sugars such as glucose, maltose, fructose and lactose can reduce the copper (II) in Benedict's solution to copper (I).
- Add Benedict's solution to glucose solution in a test tube and shake the mixture. Leave the test tube in a beaker of boiling water for five minutes.
- The colour change seen is the blue Benedict's solution turning brick-red or orange-red precipitate.
Test for starch
Iodine test:
- Starch can be detected by the iodine test. A few drops of iodine solution added to any substance containing starch will produce a blue-black colour.
Key Terms:
|
carbohydrate
proteins lipids nucleotides monomer denature |
polymer
glucose fructose galactose amino acid |
fatty acid
glycerol lactose glycogen cellulose |
starch
cholesterol unsaturated saccharide monosaccharide |
disaccharide
polysaccharide saturated denature polypetide |
Class Assignment:
Organic Molecules (ppt)
Organic Molecules _(study guide)
Saturated and Unsaturated Fats table
Useful Links:
Chem4Kids Biochemistry
BBC Bitesize Carbohydrates and Proteins
Organic Molecules (ppt)
Organic Molecules _(study guide)
Saturated and Unsaturated Fats table
Useful Links:
Chem4Kids Biochemistry
BBC Bitesize Carbohydrates and Proteins
Correct use of terminology is a key skill in Biology. It is essential to use key terms correctly when communicating your understanding, particularly in assessments. Use the quizlet flashcards or other tools such as learn, scatter, space race, speller and test to help you master the vocabulary
Video Clips:
This is a Biology tutorial dedicated to the comparison and definition of the terms Monomer and Polymer
Hank talks about the molecules that make up every living thing - carbohydrates, lipids, and proteins - and how we find them in our environment and in the food that we eat
Updated video on biomolecules (macromolecules): carbohydrates, lipids, proteins, and nucleic acids by the Amoeba Sisters including examples, functions, monomers, and structures!
Macromolecules | Classes and Functions
After a polypeptide is produced in protein synthesis, it's not necessarily a functional protein yet! Explore protein folding that occurs within levels of protein structure with the Amoeba Sisters! Primary, secondary, tertiary, and quaternary protein structure levels are briefly discussed