topic 2.1: molecules to metabolism
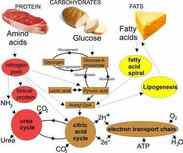 image from http://www.worldofchemicals.com/
image from http://www.worldofchemicals.com/
In the Molecules to Metabolism unit students are introduced to the major classes of biologically important molecules and the types of reactions used to build and break apart those molecules
The unit is planned to take 1 school days.
The unit is planned to take 1 school days.
Essential idea:
- Living organisms control their composition by a complex web of chemical reactions.
Nature of science:
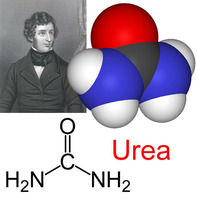
- Falsification of theories—the artificial synthesis of urea helped to falsify vitalism. (1.9)
- Define vitalism.
- Explain the role of urea in the falsification of vitalism.
- Define vitalism.
Understandings:
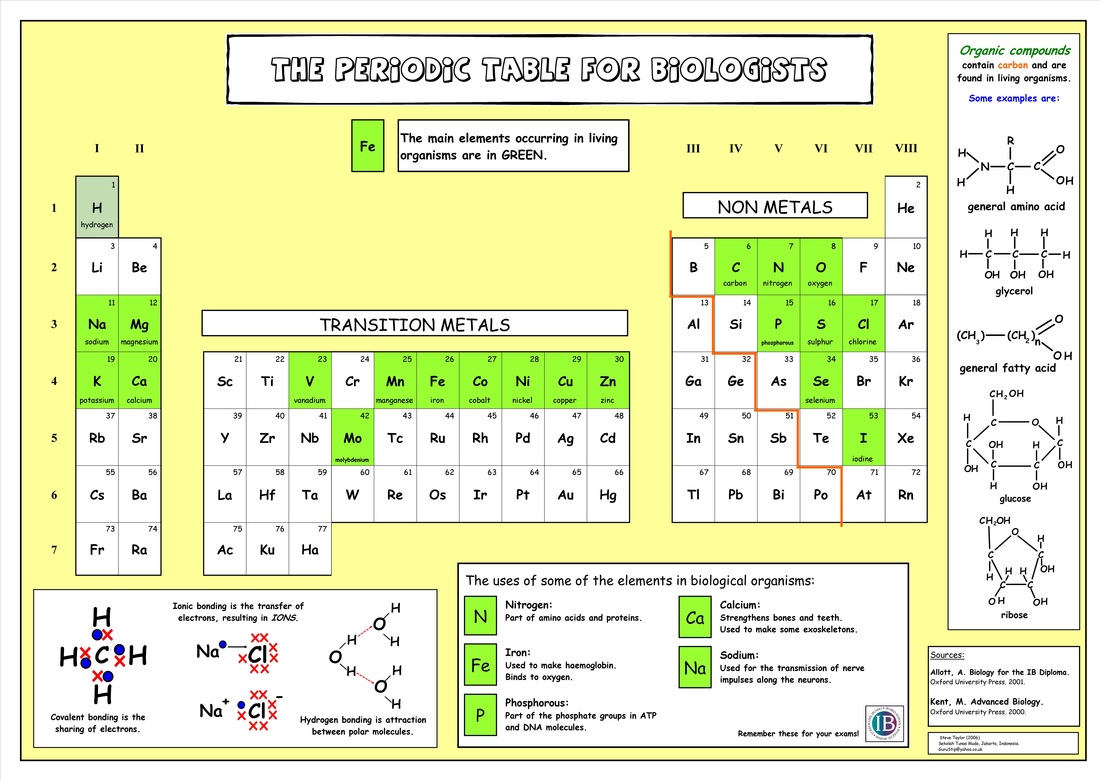
2.1 U.1 Molecular biology explains living processes in terms of the chemical substances involved (Oxford Biology Course Companion page 62).
- Define “molecular biology.”
- Compare the benefits of a reductionist and systems approach to studying biology.
- Recognize common functional groups.
- Draw skeletal molecular structures from full structure diagrams
Molecular biology involves the explaining of biological processes from the structures of the molecules and how they interact with each other. There are many molecules important to living organisms including
Biological processes are regulated by enzymes, whose expression is controlled by gene activation. The relationship between genes and proteins are important in the regulation of system functions. Molecular biologists break down biochemical processes into their component parts known as reductionism. When they look at the sum of all these reactions as a whole, they can study the emergent properties of that system.
Systems biology focuses on complex interactions within biological systems, using a more holistic perspective approach to biological and biomedical research and how these interactions give rise to the function and behavior of that system. Reductionist thinking and methods form the basis for many of the well-developed areas of modern biology. This implies the study of areas that make up smaller spatial scales and is very specific and specialized.
- water
- carbohydrates
- lipids
- proteins
- nucleic acids
Biological processes are regulated by enzymes, whose expression is controlled by gene activation. The relationship between genes and proteins are important in the regulation of system functions. Molecular biologists break down biochemical processes into their component parts known as reductionism. When they look at the sum of all these reactions as a whole, they can study the emergent properties of that system.
Systems biology focuses on complex interactions within biological systems, using a more holistic perspective approach to biological and biomedical research and how these interactions give rise to the function and behavior of that system. Reductionist thinking and methods form the basis for many of the well-developed areas of modern biology. This implies the study of areas that make up smaller spatial scales and is very specific and specialized.
2.1 U.2 Atoms can form four covalent bonds allowing a diversity of stable compounds to exist. (Oxford Biology Course Companion page 64).
- Outline the number and type of bond carbon can form with other atoms.
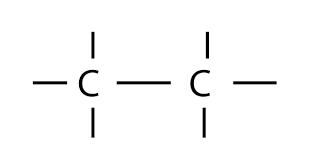
An organic compound is a compound that contains carbon and is found in living things. Carbon is very unique in its bonding properties. The most important is its ability to form long chains of carbon. No other element can bond in this way.
Carbon has an extremely carbon-carbon bond. This allows carbon to make up many of the basic building blocks of life (fats, sugars, etc). Since carbon-carbon bonds are strong and stable, carbon can form an almost infinite number of compounds
Carbon has an extremely carbon-carbon bond. This allows carbon to make up many of the basic building blocks of life (fats, sugars, etc). Since carbon-carbon bonds are strong and stable, carbon can form an almost infinite number of compounds
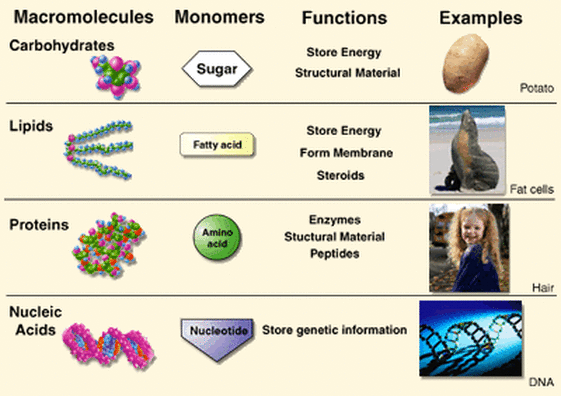
2.1 U.3 Life is based on carbon compounds including carbohydrates, lipids, proteins and nucleic acids. (Oxford Biology Course Companion page 64).
- List the four major classes of carbon compounds used by living organisms.
Main Classes of Carbon Compounds
There are four principle groups of organic compounds that contribute to much of the structure and function of a cell
Carbohydrates
Lipids
Proteins
Nucleic Acids
Roles of Elements
There are four principle groups of organic compounds that contribute to much of the structure and function of a cell
Carbohydrates
- Carbohydrates are composed of carbon, hydrogen and oxygen
- The general formula for carbohydrates is (CH2O)n.
- Many carbohydrates are used for energy or structural purposes
Lipids
- Lipids are compounds that are insoluble in water but soluble in nonpolar solvents.
- Some lipids function in long-term energy storage. Animal fat is a lipid that has six times more energy per gram than carbohydrates.
- Lipids are also an important component of cell membranes.
- Some examples of lipids are triglycerides, steroids, waxes and phospholipids
- Animal fats (saturated) are solid at room temperature and plant fats (unsaturated) are liquid at room temperature
Proteins
- Proteins are composed of one or more chains of amino acids
- All proteins are composed of carbon, hydrogen, oxygen and nitrogen
- Proteins are distinguished by their “R” groups. Some of these also contain sulfur
Nucleic Acids
- Nucleic acids are composed of smaller units called nucleotides, which are linked together to form a larger molecule (nucleic acid).
- Each nucleotide contains a base, a sugar, and a phosphate group. The sugar is deoxyribose (DNA) or ribose (RNA). The bases of DNA are adenine, guanine, cytosine, and thymine. Uracil substitutes for Thymine in RNA
- They are made from carbon, hydrogen, oxygen, nitrogen and phosphorus
Roles of Elements
- Sulphur (S): Found in certain amino acids (cysteine and methionine), allowing proteins to form disulphide bonds
- Calcium (Ca): Found in bones and teeth, also involved in neurotransmitter release in synapses
- Phosphorus (P): Component of nucleic acids and cell membranes
- Iron (Fe): Found in haemoglobin (animals), allowing for oxygen transport
- Sodium (Na): Involved in the generation of nerve impulses in neurons.
2.1 U.4 Metabolism is the web of all the enzyme-catalysed reactions in a cell or organism. (Oxford Biology Course Companion page 66)
- Define metabolism and catalysis.
- State the role of enzymes in metabolism.
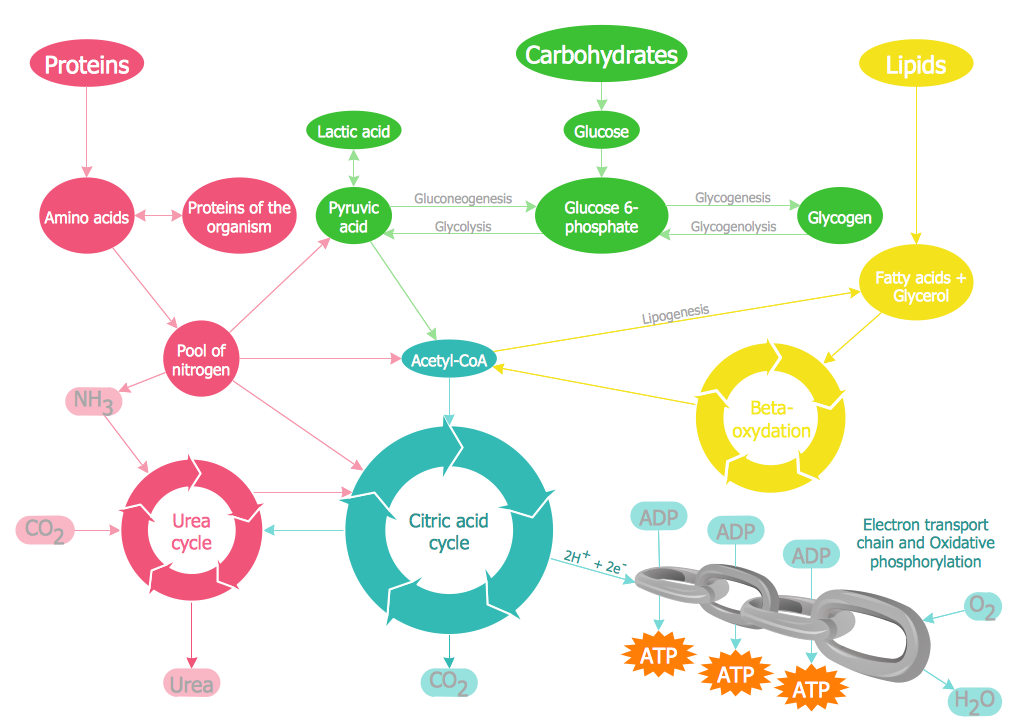
Metabolism is the sum of all chemical reactions that occur in living organisms. Metabolism describes the totality of chemical reactions that occur within a living organism in order to maintain life These reactions are catalyzed by enzymes and allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Many of these reactions occur in the cytoplasm, but some are extracellular including digestion and the transport of substances into and between different cells.
Enzymes are used to speed up the process of breaking down things (e.g.: pepsin and trypsin help break down proteins as you go through catabolic metabolism, starting from your long peptide chain all the way down to its building blocks, the amino acids.
Enzymes are used to speed up the process of breaking down things (e.g.: pepsin and trypsin help break down proteins as you go through catabolic metabolism, starting from your long peptide chain all the way down to its building blocks, the amino acids.
2.1.U5 Anabolism is the synthesis of complex molecules from simpler molecules including the formation of macromolecules from monomers by condensation reactions. (Oxford Biology Course Companion page 67)
- Define anabolism, monomer and polymer.
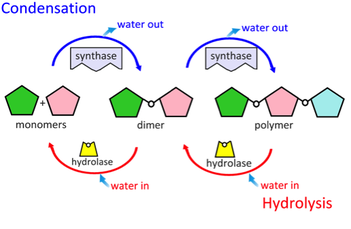
- Describe condensation (dehydration synthesis) reactions.
- Using simple shapes to represent monomers, diagram a condensation reaction.
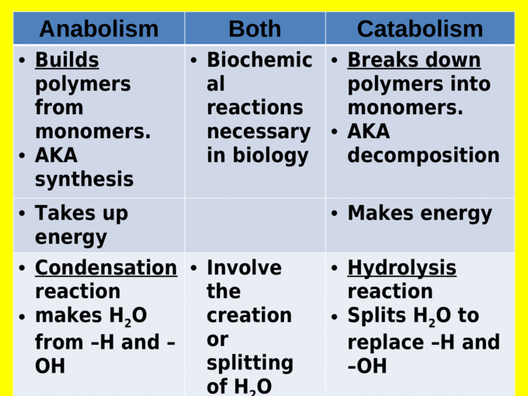
Anabolic reactions describe the set of metabolic reactions that build up complex molecules from simpler ones
The synthesis of organic molecules via anabolism typically occurs through condensation reactions
Anabolic reactions require energy as you are building large molecules from small ones (takes energy to build things). Some anabolic processes are protein synthesis,
If you can’t remember which one is which, think anabolic steroids are used to build muscles in athletes and bodybuilders and catapults are used to break down walls in wars.
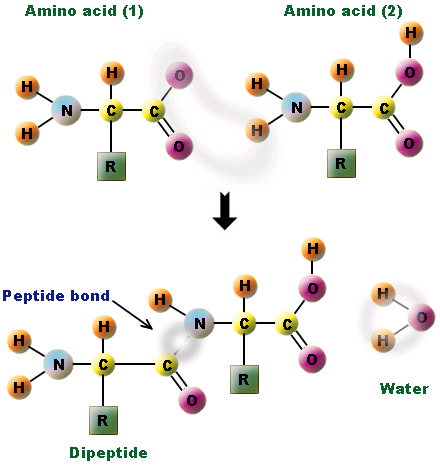
Condensation is a chemical process by which 2 molecules are joined together to make a larger, more complex, molecule, with the loss of water. It is the basis for the synthesis of all the important biological macromolecules (carbohydrates, proteins, lipids, nucleic acids) from their simpler sub-units.
In all cases of condensation, molecules with projecting -H atoms are linked to other molecules with projecting -OH groups, producing H2O, ( H.OH ) also known as water, which then moves away from the original molecules.
The synthesis of organic molecules via anabolism typically occurs through condensation reactions
Anabolic reactions require energy as you are building large molecules from small ones (takes energy to build things). Some anabolic processes are protein synthesis,
- Monosaccharides are joined via glycosidic linkages to form disaccharides and polysaccharides
- Amino acids are joined via peptide bonds to make polypeptide chains
- Glycerol and fatty acids are joined via an ester linkage to create triglycerides
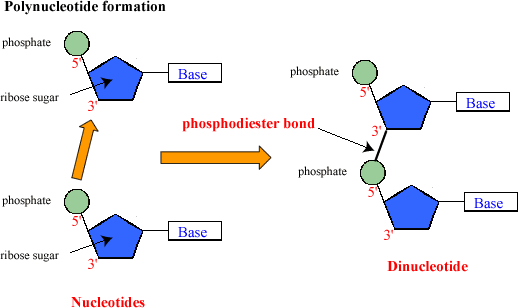
- Nucleotides are joined by phosphodiester bonds to form polynucleotide chains
If you can’t remember which one is which, think anabolic steroids are used to build muscles in athletes and bodybuilders and catapults are used to break down walls in wars.
Condensation is a chemical process by which 2 molecules are joined together to make a larger, more complex, molecule, with the loss of water. It is the basis for the synthesis of all the important biological macromolecules (carbohydrates, proteins, lipids, nucleic acids) from their simpler sub-units.
In all cases of condensation, molecules with projecting -H atoms are linked to other molecules with projecting -OH groups, producing H2O, ( H.OH ) also known as water, which then moves away from the original molecules.
2.1.U6 Catabolism is the breakdown of complex molecules into simpler molecules including the hydrolysis of macromolecules into monomers. (Oxford Biology Course Companion page 67).
- Define catabolism.
- Contrast anabolism and catabolism.
- Describe hydrolysis reactions.
- Using simple shapes to represent monomers, diagram a hydrolysis reaction.
Catabolism is a metabolic reaction that break down larger molecules into smaller ones or their component parts
Catabolic reactions release energy which can sometimes be captured in the form of ATP. The breakdown of organic molecules through catabolism typically occurs through the hydrolysis reactions
Hydrolysis reactions require the consumption of water molecules to break the bonds within the polymer
Examples of catabolic reactions are digestion of food, cellular respiration, and break down of carbon compounds by decomposers
Catabolic reactions release energy which can sometimes be captured in the form of ATP. The breakdown of organic molecules through catabolism typically occurs through the hydrolysis reactions
Hydrolysis reactions require the consumption of water molecules to break the bonds within the polymer
Examples of catabolic reactions are digestion of food, cellular respiration, and break down of carbon compounds by decomposers
Applications:
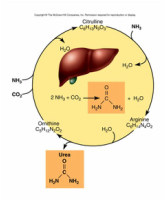
2.1.A1 Urea as an example of a compound that is produced by living organisms but can also be artificially synthesized. (Oxford Biology Course Companion page 62).
- Draw the molecular structure of urea.
- Describe how urea can be synthesized by living and artificial mechanisms.
Urea is a component of urine which is produced when there is an excess of amino acids in the body; way to secrete nitrogen. A series of enzyme catalyzed reactions produce urea in the liver, where it is transported by the blood to the kidney, where it is filtered out and excreted in the urine.
Urea can be produced artificially through different chemical reactions; however, the product is the same.
Urea is mainly used as a nitrogen source in fertilizer
Urea can be produced artificially through different chemical reactions; however, the product is the same.
Urea is mainly used as a nitrogen source in fertilizer
In 1828, Friedrich Wöhler, a German physician and chemist by training, published a paper that describes the formation of urea, known since 1773 to be a major component of mammalian urine, by combining cyanic acid and ammonium in vitro. In these experiments the synthesis of an organic compound from two inorganic molecules was achieved for the first time. These results weakened significantly the vitalistic hypothesis on the functioning of living cells, Vitalism was a doctrine that dictated that organic molecules could only be synthesised by living systems. It was believed that living things possessed a certain “vital force” needed to make organic molecules. Organic compounds were thought to possess a non-physical element lacking from inorganic molecules.
Skills:
2.1.S1 Drawing molecular diagrams of glucose, ribose, a saturated fatty acid and a generalized amino acid. (Oxford Biology Course Companion page 65).
- Draw the molecular diagram of ribose.
- Draw the molecular diagram of alpha-glucose.
- Draw the molecular diagram of a saturated fatty acid.
- Identify the carboxyl and methyl groups on a fatty acid.
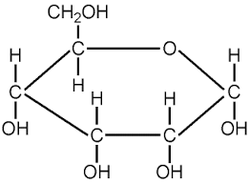
Glucose
- Is a reducing sugar that contains C6H12O6
- Most commonly found in a ringed structure and is the main product formed by photosynthesis
- Energy molecule used in aerobic respiration
- Monomer of starch, glycogen, and cellulose
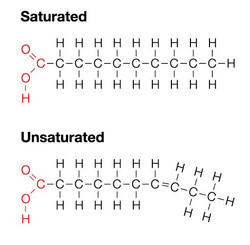
Fatty Acids
- Main component of triglycerides and phospholipids
- Fatty acids are non-polar and therefore hydrophobic
- Chains consist of covalently bonded carbon with hydrogen
- Saturated fatty acid’s are all single bonds and are therefore saturated with hydrogen.
- Unsaturated fatty acid’s contain a double bond or double bonds.
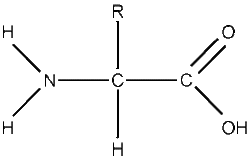
Amino Acid
- Composed of an amine (NH2) group, a carboxyl (COOH) group, and an R group.
- 20 amino acids exist that compose all proteins
- Each amino acid differs because the R groups are different
2.1.S2 Identification of biochemical such as sugars, lipids, or amino acids from molecular drawings.(Oxford Biology Course Companion page 66).
- Identify the four major classes of carbon compounds used by living organisms from given diagrams (examples will include D-ribose, alpha glucose, beta glucose, triglycerides, phospholipids and steroids).
- State the generalized chemical formula of the carbohydrates. Identify the following carbohydrates from molecular
- drawings.
- D-ribose
- alpha glucose
- beta glucose
- cellulose
- glycogen
- amylose starch
- amylopectin starch
- Compare the relative amount of oxygen atoms in lipids to the amount in carbohydrates.
- Identify the following lipids from molecular drawings.
- Triglycerides
- phospholipids
- steroids
- Triglycerides
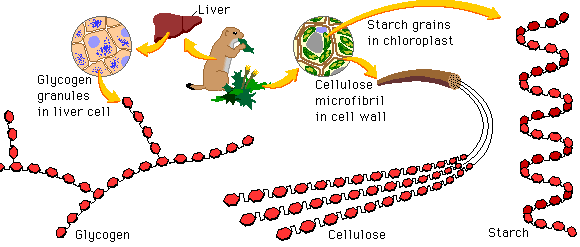
Carbohydrates
The structure of complex carbohydrates may vary depending on the composition of monomeric subunits.
Polysaccharides may differ according to the type of monosaccharide they possess and the way the subunits bond together. The generalized formula for carbohydrates is CH2O. All carbohydrate contain C, H and O
Glucose monomers can be combined to form a variety of different polymers – including glycogen, cellulose and starch
The structure of complex carbohydrates may vary depending on the composition of monomeric subunits.
- Glucose
- Fructose
- Galactose
Polysaccharides may differ according to the type of monosaccharide they possess and the way the subunits bond together. The generalized formula for carbohydrates is CH2O. All carbohydrate contain C, H and O
Glucose monomers can be combined to form a variety of different polymers – including glycogen, cellulose and starch
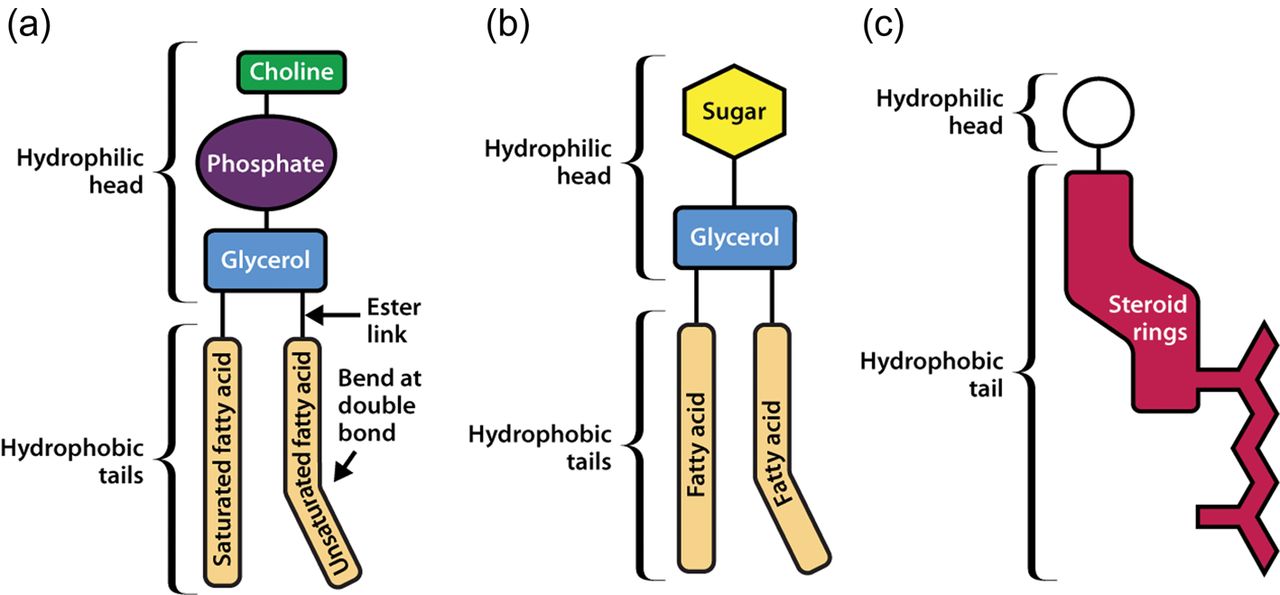
Lipids
Lipids contain C, H and O as well, but in different ratios and much less O then carbohydrates.
Lipids can be roughly organised into one of three main classes:
Lipids contain C, H and O as well, but in different ratios and much less O then carbohydrates.
Lipids can be roughly organised into one of three main classes:
- a. Compound lipids – Esters of fatty acids, alcohol and additional groups (e.g. phospholipids and glycolipids)
- b. Simple (neutral) lipids – Esters of fatty acids and alcohol (e.g. triglycerides and waxes)
- c. Derived lipids – Substances derived from simple or compound lipids (e.g. steroids and carotenoids)
Proteins
Amino acids join together by peptide bonds which form between the amine and carboxyl groups of adjacent amino acids. Proteins also contain C,H, O but they all have N. Some proteins also contain S in their R-groups
The fusion of two amino acids creates a dipeptide, with further additions resulting in the formation of a polypeptide chain. The subsequent folding of the chain depends on the order of amino acids in a sequence (based on chemical properties)
Amino acids join together by peptide bonds which form between the amine and carboxyl groups of adjacent amino acids. Proteins also contain C,H, O but they all have N. Some proteins also contain S in their R-groups
The fusion of two amino acids creates a dipeptide, with further additions resulting in the formation of a polypeptide chain. The subsequent folding of the chain depends on the order of amino acids in a sequence (based on chemical properties)
Nucleic Acids
There are two varieties: ribonucleic acid, or RNA, and deoxyribonucleic acid, or DNA. These polymers are long chains of components called bases, of which there are only five types. RNA is a single-strand molecule; DNA is a spiral of two cross-connected strands.
Nucleotides form bonds between the pentose sugar and phosphate group to form long polynucleotide chains.
In DNA, two complementary chains will pair up via hydrogen bonding between nitrogenous bases to form double strands. This double stranded molecule may then twist to form a double helical arrangement
There are two varieties: ribonucleic acid, or RNA, and deoxyribonucleic acid, or DNA. These polymers are long chains of components called bases, of which there are only five types. RNA is a single-strand molecule; DNA is a spiral of two cross-connected strands.
Nucleotides form bonds between the pentose sugar and phosphate group to form long polynucleotide chains.
In DNA, two complementary chains will pair up via hydrogen bonding between nitrogenous bases to form double strands. This double stranded molecule may then twist to form a double helical arrangement
Key Terms:
|
fatty acid
element trace element matter proton neutron electron metabolic reaction coolant vaporization catalyst steroids beta glucose |
covalent
polar ionic ion cation carbohydrate lipid polarity thermal enzyme carboxyl group methyl groups cellulose |
anion
hydrogen bond organic monomer polymer carbon hydrogen sodium iron functional groups amino acid phospholipids glycogen |
condensation
hydrolysis hydrophobic hydrophilic adhesion oxygen nitrogen phosphorous anabolism dehydration R-group triglycerides amylose starch |
density
nucleic acid protein sulfur calcium vitalism reductionist approach systems approach catabolism alpha-glucose D-ribose carbohydrate amylopectin starch |
Classroom Material:
Chemistry Basics
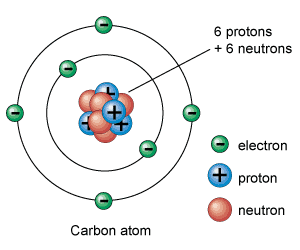
Atomic Structure
Common Elements
Chemical Bonds.
Chemical bonds (worksheet)
Biochemistry Posters
Chemistry reading guide
Basic of Biochemistry (ppt)
Basics of Biochemistry (notes)
Chemical Bonding review
Topic 2.1 Review notes
Topic 2.1 Kahoot Review Quiz
Chemistry Basics
Atomic Structure
Common Elements
Chemical Bonds.
Chemical bonds (worksheet)
Biochemistry Posters
Chemistry reading guide
Basic of Biochemistry (ppt)
Basics of Biochemistry (notes)
Chemical Bonding review
Topic 2.1 Review notes
Topic 2.1 Kahoot Review Quiz
Powerpoint and Notes on Topic 2.1 from Chris Payne
Your browser does not support viewing this document. Click here to download the document.
Your browser does not support viewing this document. Click here to download the document.
Correct use of terminology is a key skill in Biology. It is essential to use key terms correctly when communicating your understanding, particularly in assessments. Use the quizlet flashcards or other tools such as learn, scatter, space race, speller and test to help you master the vocabulary.
Useful Links:
Biochemistry
The macromolecules of life
The Chemical Basis of Life
Lipid Dehydration
Dehydration Synthesis and Hydrolysis
Periodic Table Interactive
Drag and Drop Molecules
Interactive Chemistry Quiz
Four Types of Chemical Reactions
Animated Bonds
In the News:
Researchers take small step toward silicon-based life (2016-03-18)
Video Clips:
Infographics animated video simplifying the role of Systems Biology in biological research. produced for the Weizmann Institute of Science
And thus begins the most revolutionary biology course in history. Come and learn about covalent, ionic, and hydrogen bonds. What about electron orbitals, the octet rule, and what does it all have to do with a mad man named Gilbert Lewis? It's all contained within
Hank talks about the molecules that make up every living thing - carbohydrates, lipids, and proteins - and how we find them in our environment and in the food that we eat.
Paul Andersen describes the four major biological molecules found in living things. He begins with a brief discussion of polymerization. Dehydration synthesis is used to connect monomers into polymers and hydrolysis breaks them down again. The major characteristics of nucleic acids are described as well as there directionality from 3' to 5' end. Protein structure is describes as well as the structure of its monomers; amino acids. The carboxyl and amino ends of a protein are described. The major groups of lipids are included with a brief discussion of saturated, unsaturated and trans fats. Finally carbohydrates and their sugar monomers are discussed.
Paul Andersen describes the macromolecules that make up living organisms. He starts with a brief description of organic chemistry and the importance of functional groups. He also covers both dehydration and hydrolysis in polymerization. He finally covers the four major macromolecules: nucleic acids, proteins, lipids and carbohydrates.
Updated video on biomolecules (macromolecules): carbohydrates, lipids, proteins, and nucleic acids by the Amoeba Sisters including examples, functions, monomers, and structures.
Paul Andersen begins by explaining the structure and purpose of carbohydrates. He describes and gives examples of monosaccharides, disaccharides, oligosaccharide and polysaccharides. He explains how they grow through dehydration reactions and shrink through hydrolysis
In this video Paul Andersen describes the lipids (of the fats). He explains how they are an important source of energy but are also required to cell membranes. He explains how the hydrocarbon tails in triglycerides contain energy available for life. He also explains how phospholipids construct, and cholesterol molecules main the cell membrane.
Paul Andersen explains the structure and importance of proteins. He describes how proteins are created from amino acids connected by dehydration synthesis. He shows the importance of chemical properties in the R-groups of individual amino acids in the polypeptide. He explains the four levels of protein folding and gives you an opportunity to fold proteins of your own using the game Foldit:
Paul Andersen explains the importance and structure of nucleic acids. He begins with an introduction to DNA and RNA. He then describes the important parts of a nucleotide and shows how they are connected through covalent and hydrogen bonding.
In 1828, Fredric Wohler heated an inorganic salt (ammonium cyanate) and produced urea