Topic 8.2: Cell Respiration
In the Cell Respiration unit we will expand our knowledge of Topic 2.8. We will explore the structure of the mitochondria and identify how it is adapted to produce energy. We will also identify specific metabolic pathways of Glycolysis and the Kreb Cycles
This unit will last 5 school days
This unit will last 5 school days
Essential idea:
- Energy is converted to a usable form in cell respiration
Nature of science:
- Paradigm shift—the chemiosmotic theory led to a paradigm shift in the field of bioenergetics. (2.3)
- State that Peter Mitchell’s proposal of the chemiosmotic hypothesis in 1961 lead to a major shift in our understanding of cellular processes.
- State that Peter Mitchell’s proposal of the chemiosmotic hypothesis in 1961 lead to a major shift in our understanding of cellular processes.
Understandings
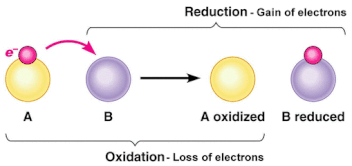
8.2 U 1 Cell respiration involves the oxidation and reduction of electron carriers.
- Outline oxidation and reduction reactions in terms of movement of electrons, hydrogen or oxygen atoms.
- Define “electron carrier.”
- State the name of the electron carrier molecule used in cellular respiration
Cell respiration is the controlled release of energy from organic compounds to produce ATP. Anaerobic respiration involves the incomplete breakdown of organic molecules for a small yield of ATP (no oxygen required). Aerobic respiration involves the complete breakdown of organic molecules for a larger yield of ATP (oxygen is required)
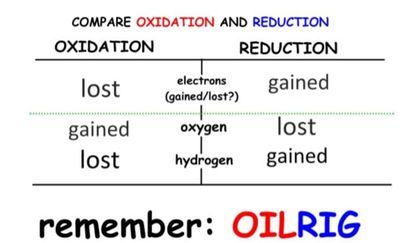
- Oxidation involves the loss of electrons from an element through the gain of oxygen or the loss of hydrogen.
- Reduction involves the gain of electrons through the addition of hydrogen or the loss of an oxygen molecule.
- These reactions are called Redox reactions (reduction-oxidation) which are chemical reactions in which atoms have their oxidation number changed.
- For example the oxidation of carbon to form CO2 and the reduction of carbon by the addition of hydrogen to yield methane (CH4).
- Electron carriers are specific substances that accept and give up electrons
- The main electron carrier in cellular respiration is NAD (nicotinamide adenine dinucleotide)
- During respiration NAD which actually exists as NAD+ accepts 2 electrons and a proton (H+) from the molecule being oxidized (like pyruvate) to form NADH with one extra H+ leftover as a product.
- After the electron carriers are reduced they transport their electrons and hydrogens to the ETC, where the opposite reaction occurs (oxidation)
- So when NADH is oxidized it donates the electrons and protons to an electron carrier (complex I) in the inner mitochondrial membrane made from conjugated proteins (Fe-S core)
- This carrier is therefore reduced and will be re-oxidized as it passes the electrons down the ETC
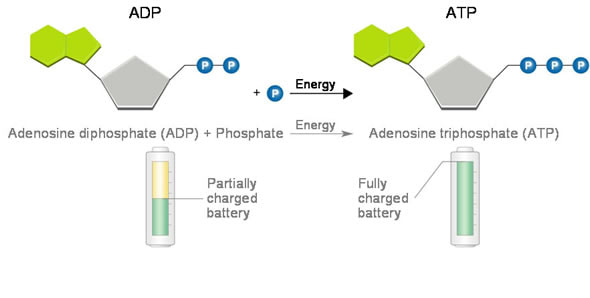
8.2 U 2 Phosphorylation of molecules makes them less stable
- Define phosphorylation.
- State the consequence of a molecule being phosphorylated.
Adenosine triphosphate (ATP) is a high energy molecule that functions as an immediate power source for cells
- Phosphorylation occurs when a phosphate (PO43-) molecule is added to an organic molecule to make the molecule less stable and more likely to react
- Phosphorylation basically activates the molecule and is an endergonic reaction
- The removal of the phosphate through hydrolysis is an exergonic reaction
- The reactions are coupled together, so one molecule releases the phosphate and one accepts the phosphate molecule, and are spontaneous
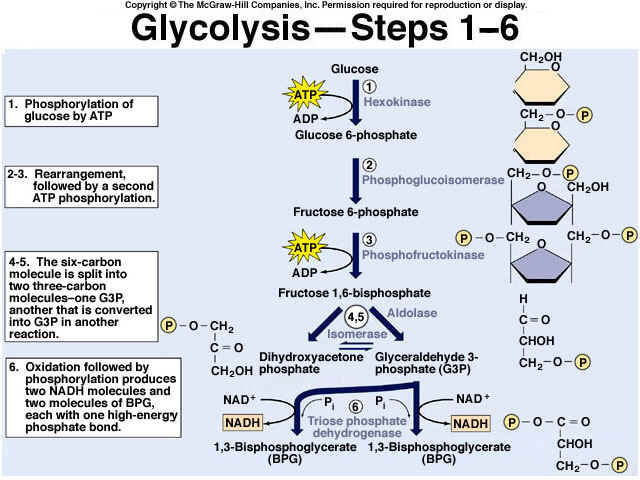
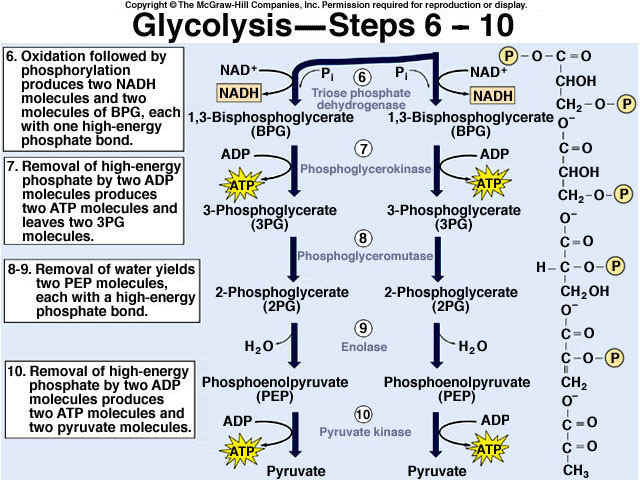
8.2 U 3 In glycolysis, glucose is converted to pyruvate in the cytoplasm.
- Outline the glycolysis reaction, including phosphorylation, lysis and energy harvest.
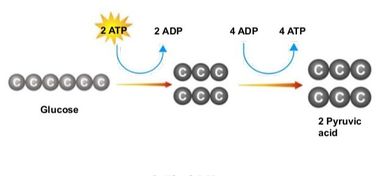
Glycolysis is the process of releasing energy within sugars. In glycolysis, glucose (a six carbon sugar) is split into two molecules of the three-carbon sugar pyruvate. This multi-step process yields two molecules of ATP (free energy containing molecule), two molecules of pyruvate, and two "high energy" electron carrying molecules of NADH. Glycolysis can occur with or without oxygen
Glycolysis: Energy generation phase
8.2 U 4 Glycolysis gives a small net gain of ATP without the use of oxygen.
- State the formula for the glycolysis reaction.
- State that glycolysis occurs in both anaerobic and aerobic respiration.
- State that glycolysis is an example of a metabolic pathway.
Glycolysis involves the breakdown of glucose into pyruvate (× 2), with a small net gain of ATP (two molecules). This occurs in the cytosol and does not require oxygen (it is an anaerobic process). Phosphorylation of glucose requires 2 ATP molecules. The phosphorylation of molecules makes them less stable.
Net gain: 2 ATP, 2 NADH + H+, 2 pyruvate
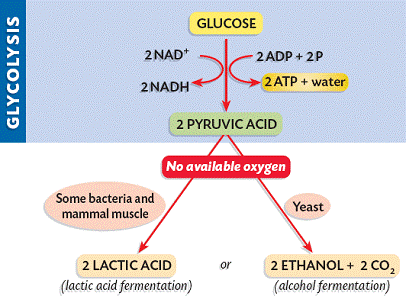
Depending on the availability of oxygen, the pyruvate may be subjected to one of two alternative processes:
If oxygen is not present, pyruvate is not broken down further and no more ATP is produced (incomplete oxidation). The pyruvate remains in the cytosol and is converted into lactic acid (animals) or ethanol and CO2 (plants and yeast).
This conversion is reversible and is necessary to ensure that glycolysis can continue to produce small quantities of ATP
Net gain: 2 ATP, 2 NADH + H+, 2 pyruvate
Depending on the availability of oxygen, the pyruvate may be subjected to one of two alternative processes:
- Aerobic respiration occurs in the presence of oxygen and results in the further production of ATP (~ 34 molecules)
- Anaerobic respiration (fermentation) occurs in the absence of oxygen and no further ATP is produced
If oxygen is not present, pyruvate is not broken down further and no more ATP is produced (incomplete oxidation). The pyruvate remains in the cytosol and is converted into lactic acid (animals) or ethanol and CO2 (plants and yeast).
This conversion is reversible and is necessary to ensure that glycolysis can continue to produce small quantities of ATP
- Glycolysis involves oxidation reactions that cause hydrogen carriers (NAD+) to be reduced (becomes NADH + H+)
- Typically, the reduced hydrogen carriers are oxidised via aerobic respiration to restore available stocks of NAD+
- In the absence of oxygen, glycolysis will quickly deplete available stocks of NAD+, preventing further glycolysis
- Fermentation of pyruvate involves a reduction reaction that oxidises NADH (releasing NAD+ to restore available stocks)
- Hence, anaerobic respiration allows small amounts of ATP to be produced (via glycolysis) in the absence of oxygen
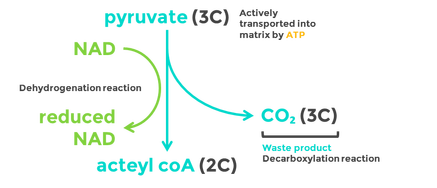
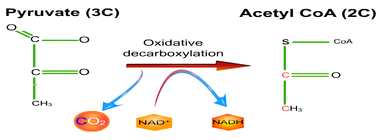
8.2 U 5 In aerobic cell respiration pyruvate is decarboxylated and oxidized, and converted into acetyl compound and attached to coenzyme A to form acetyl coenzyme A in the link reaction.
- Define decarboxylation and oxidation.
- Summarize the reactant and products of the link reaction.
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2).
Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion. Pyruvate is oxidized by the by the removal of pairs of hydrogen atoms (with their electrons), which are passed on the NAD+ and FAD
Link Reaction
Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion. Pyruvate is oxidized by the by the removal of pairs of hydrogen atoms (with their electrons), which are passed on the NAD+ and FAD
Link Reaction
- Two pyruvate molecules enter the matrix of the mitochondria and are decarboxylated to form two acetyl groups (removal of carbon as CO2).
- The acetyl group is oxidized and NAD+ is reduced to form NADH.
- Each acetyl group combines with CoA (enzyme) producing 2 Acetyl Coenzyme A molecules.
- These products enter the Krebs cycle
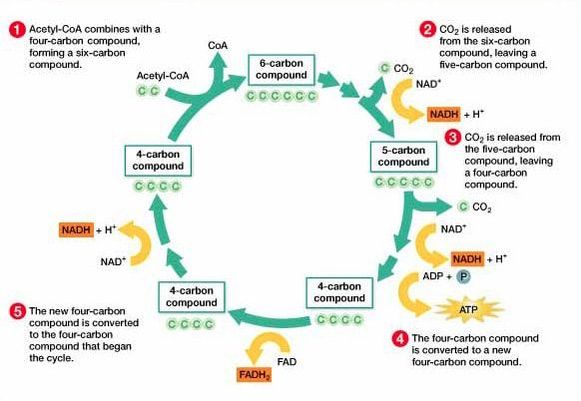
8.2 U 6 In the Krebs cycle, the oxidation of acetyl groups is coupled to the reduction of hydrogen carriers, liberating carbon dioxide.
- State that NADH and FADH2 are electron carriers formed during the Krebs cycle.
- Outline the events of the Krebs cycle, referencing the formation of NADH and FADH2, formation of ATP and decarboxylation of acetyl groups.
Each acetyl group enters the Krebs cycle, therefore there are two rotations of the Krebs cycle per glucose molecule. There are two decarboxylations and four oxidations per cycle**
The carbon dioxide that is removed in these reactions is a waste product and is excreted from the body. The oxidations release energy which is then stored by the carriers when they accept the hydrogen. This energy is then later on used by the electron transport chain to produce ATP.
- Step 1 - In the first stage of the Krebs cycle, the acetyl group from acetyl CoA is transferred to a four carbon compound. This forms a six carbon compound.
- Step 2 - This six carbon compound then undergoes decarboxylation (CO2 is removed) and oxidation (hydrogen is removed) to form a five carbon compound. The hydrogen is accepted by NAD+ and forms NADH + H+.
- Step 3 - The five carbon compound undergoes decarboxylation and oxidation (hydrogen is removed) again to form a four carbon compound. The hydrogen is accepted by NAD+ and forms NADH + H+.
- Step 4 - The four carbon compound then undergoes substrate-level phosphorylation and during this reaction it produces ATP. Oxidation also occurs twice (2 hydrogens are removed). The one hydrogen is accepted by NAD+ and forms NADH + H+. The other is accepted by FAD and forms FADH2. The four carbon compound is then ready to accept a new acetyl group and the cycle is repeated.
The carbon dioxide that is removed in these reactions is a waste product and is excreted from the body. The oxidations release energy which is then stored by the carriers when they accept the hydrogen. This energy is then later on used by the electron transport chain to produce ATP.
8.2 U 7 Energy released by oxidation reactions is carried to the cristae of the mitochondria by reduced NAD and FAD.
- State that NAD+ is reduced to become NADH in the link reaction and Krebs cycle.
- State that FAD is reduced to become FADH2 in the Krebs cycle.
- State that NADH and FADH2 carry electrons to the electron transport chain on the mitochondrial inner membrane.
Energy released from the oxidation of the acetyl groups is transferred to electron carriers NAD and FAD to form NADH and FADH2. This energy is carried by these two molecules to the cristae of the mitochondria
The energy transferred is used to create ATP through a process called oxidative phosphorylation
The energy transferred is used to create ATP through a process called oxidative phosphorylation
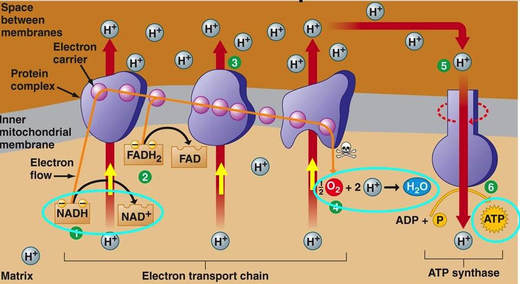
8.2 U 8 Transfer of electrons between carriers in the electron transport chain in the membrane of the cristae is coupled to proton pumping.
- State that at the electron transport chain, FADH2 and NADH given electrons to electron carrier proteins.
- State that the movement of electrons through electron carrier proteins in the electron transport chain is used to pump protons (H+) across the inner mitochondrial membrane into the intermembrane space.
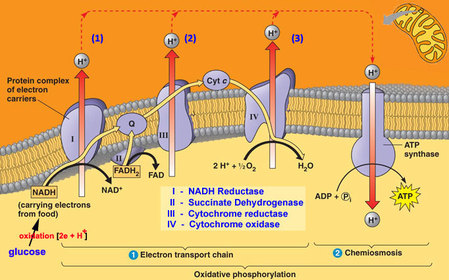
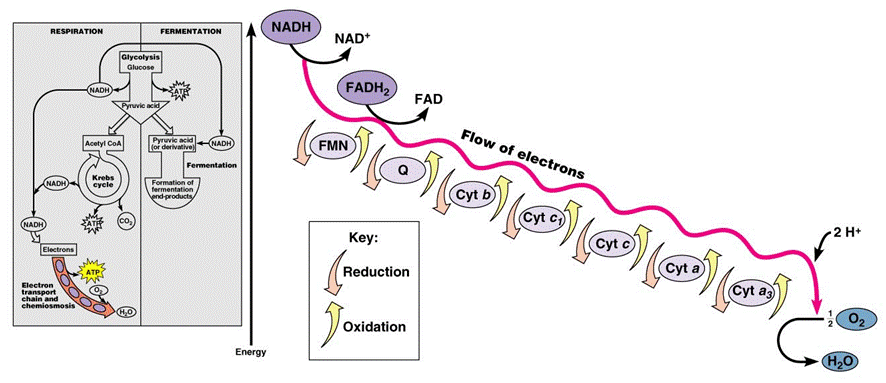
In the mitochondria, there is a chain of electron carriers, which is best known as the electron transport chain. It is through this chain that electrons from the oxidative reactions before pass along the chain. NADH donates two electrons to the first carrier in the chain. Then, these two electrons go along the chain releasing energy from one carrier to the next. At the third location of the chain, enough energy has been released to produce ATP with the usage of the ATP synthase, which is an enzyme that is found in the inner mitochondrial membrane as well. FADH2 donates electrons but at later stage than NADH. Enough energy is released at only two locations of the chain by electrons from FADH2. The ATP production relies on the released energy by oxidation and it is thus called oxidative phosphorylation
Electron transport chain (ETS):
Electron transport chain (ETS):
- mitochondrial inner membrane proteins of the cristae form a transport chain for electrons and protons
- NADH + H+ arrives at the first carrier and transfers 2 e-s and 1 H+
- another H+ is picked up from the matrix solution
- the 2 e-s and 2 H+s are carried from the inner to outer face,
- where the 2 H+s are deposited in the intermembrane space;
- the 2 e-s return, pick up another pair of H+s and repeat the trip 2 more times,
- for a total of 3 round trips;
- FADH2 enters into the ETS further along,
- and thus pumps only 2 pairs of H+s into the intermembrane space
8.2 U 9 In chemiosmosis protons diffuse through ATP synthase to generate ATP.
- Define oxidative phosphorylation and chemiosmosis.
The chain is a series of electron carriers located in the inner membrane of mitochondria that pass electrons from one carrier to the next down an energy gradient.
- NADH supplies 2 electrons to the first carrier in the chain (reforming NAD+). These electrons move along the chain of electron carriers giving up energyeach time they pass from one carrier to the next.
- FADH2 is also oxidized (forms FAD) releases its electrons a little later into the electron transport chain.
- Energy is released as the electrons are passed along the carrier proteins.
- This energy is used to pump H+ ions across the inner mitochondrial membrane from the matrix to the intermembrane space.
- This accumulation of H+ ions in the intermembrane space creates an H+concentration gradient.
- Protons (H+) flow back from the intermembrane space to the matrix throughspecial protein channels located in the inner mitochondrial membrane calledATP synthase.
- As the protons pass across the membrane, they release energy, which is usedby the ATP synthase to produce ATP through a phosphorylation reaction.
- This process is called oxidative phosphorylation because oxygen is the final electron acceptor and the energy released by reducing oxygen to water is used to phosphorylate ADP and generate ATP.
- For each glucose molecule, about 32 molecules of ATP are produced.
8.2 U 10 Oxygen is needed to bind with the free protons to maintain the hydrogen gradient, resulting in the formation of water.
- State that oxygen is the final electron acceptor in aerobic cellular respiration.
- State that that formation of water in the matrix at the end of the electron transport chain helps to maintain the hydrogen gradient between the intermembrane space and the matrix.
At the end of the ETC, electrons are given to oxygen. Oxygen accepts hydrogen ions and forms water (known as the terminal acceptor).
- If oxygen is not available, electron flow along the ETC stops and NADH + H+cannot be reconverted to NAD+.
- Supplies of NAD+ in the mitochondrion run out and the link reaction and Krebs cycle cannot continue.
- Glycolysis can continue because conversion of pyruvate into lactate or ethanol and carbon dioxide produces as much NAD+ as is used in glycolysis.
- Therefore oxygen is necessary for aerobic respiration to take place.
- Also, by using up the hydrogen to form water, the proton gradient across the inner mitochondrial membrane is maintained.
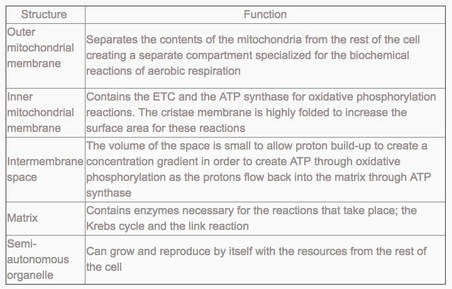
8.2 U 11 The structure of the mitochondrion is adapted to the function it performs.
- Outline how mitochondria structure could evolve through natural selection.
- State evidence that suggests mitochondria were once free living prokaryotes.
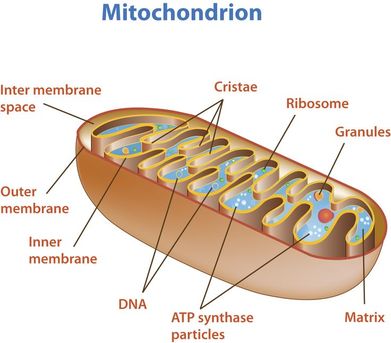
outer membrane:
- impermeable to H+s,
- facilitates diffusion of pyruvate,
- shuttles 2 e-s from glycolytic NADH + H+ to inside of mitochondrion
- low pH = high concentration of H+s from ETS/proton pump
- folded into cristae, increasing SA for ETS and ATP synthetase;
- permeable to H+s
- contains enzymes for oxidative decarboxylation and Krebs cycle
Application
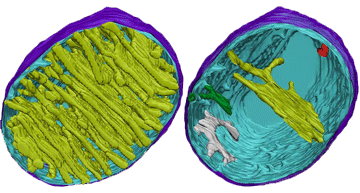
8.2 A 1 Electron tomography used to produce images of active mitochondria
- State that electron tomography enables scientists to view the dynamic nature of mitochondrial membranes.
Electron tomography is a technique by which the 3-dimensional internal structure of a sample can be modeled
Used to create a 3-D image of active mitochondria
Show cristea are connected to intermembrane space
Show shape and volume changes of cristae in active mitochondria.
Used to create a 3-D image of active mitochondria
Show cristea are connected to intermembrane space
Show shape and volume changes of cristae in active mitochondria.
Skills
8.2 S 1 Analysis of diagrams of the pathways of aerobic respiration to deduce where decarboxylation and oxidation reactions occur. (Guidance: The names of the intermediate compounds in glycolysis and the Krebs cycle are not required)
- State that decarboxylation of glucose occurs in the linking reaction and Krebs cycle of aerobic respiration.
Aerobic respiration involves the breakdown of glucose in the presence of oxygen to produce water and carbon dioxide
Aerobic respiration involves three main types of reactions – decarboxylation, oxidation and phosphorylation
Aerobic respiration involves three main types of reactions – decarboxylation, oxidation and phosphorylation
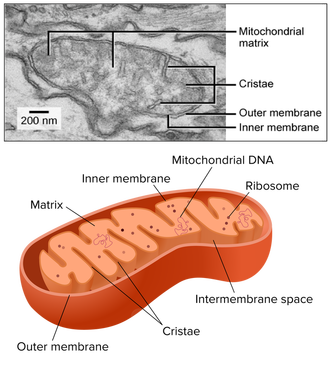
8.2 S 2 Annotation of a diagram of a mitochondrion to indicate the adaptations to its function.
- Draw and label a diagram of the mitochondria.
- State the function of the following mitochondrial structures: outer membrane, inner membrane, cristae, intermembrane space, matrix, ribosome and mtDNA.
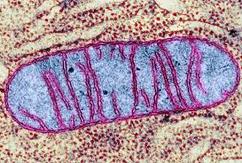
Electron micrographs of a mitochondrion may differ in appearance depending on where the cross-section occurs
Typically, mitochondrial diagrams should display the following features:
Typically, mitochondrial diagrams should display the following features:
- Usually sausage-shaped in appearance (though will appear more rounded in perpendicular cross-sections)
- Inner membrane contains many internal protrusions (cristae)
- Intermembrane space is very small (allows for a more rapid generation of a proton motive force)
- Ribosomes and mitochondrial DNA are usually not visible at standard resolutions and magnifications
Key Terms
|
oxidation
NADH decarboxylated acetyl groups protons lysis decarboxylated hydrogen carriers hydrogen gradient |
reduction
glucose link reaction citric acid chemiosmosis energy harvest coenzyme A proton pump bioenergetics |
phosphorylation
ATP acetyl coenzyme A FAD electron transport chain anaerobic acetyl coenzyme A electron transport chain electron tomography |
electron carriers
glycolysis FADH aerobic Krebs cycle cristae mtDNA |
NAD
pyruvate mitochondria oxidative phosphorylation ATP synthase pyruvate link reaction NADPH Peter Mitchell |
PowerPoint and Notes on Topic 8.2 by Chris Payne
Your browser does not support viewing this document. Click here to download the document.
Your browser does not support viewing this document. Click here to download the document.
Correct use of terminology is a key skill in Biology. It is essential to use key terms correctly when communicating your understanding, particularly in assessments. Use the quizlet flashcards or other tools such as learn, scatter, space race, speller and test to help you master the vocabulary.
Useful Links
Cellular Respiration
Metabolism Problem Set
Mitochondria
ATP Synthesis in Mitochondria Animation
In The News
Key Protein In Cellular Respiration Discovered - Science Daily, Apr 2009
Breakthrough in understanding mitochondria - Science Daily, Aug 2017
TOK
- Peter Mitchell’s chemiosmotic theory encountered years of opposition before it was finally accepted. For what reasons does falsification not always result in an immediate acceptance of new theories or a paradigm shift?
Video Clips
Explore how ATP is made in 3 steps of aerobic cellular respiration with the Amoeba Sisters!
You need energy to do literally anything, even just lay still and think. Where does this energy come from? Well, food, right? But how? This is one of the most miraculous biological processes, and it happens in our bodies every moment of every day. It's quite complicated, so let's check it out in three sections.
Sequels usually suck, but this one is really good. Nature figured out a way to improve on glycolysis by stumbling upon the citric acid cycle and oxidative phosphorylation, so let's look at the first of those two in this clip.
The trilogy is finally concluded! And with a bang, no less, as this final stage of cellular respiration is the one that provides the big energy payoff for the cell. Let's learn about how the products of the Citric Acid cycle go on to the electron transport chain to facilitate reactions that will generate a proton gradient suitable for powering ATP synthase, one of the most fascinating proteins in the body
How does your body break down the food you eat to generate the energy you need to get through your day? What form of energy is this, anyway? Let's go over some of the basics of metabolic processes, and introduce ATP, the currency of cellular energy.
This tutorial is the first in the Cellular Respiration series. This tutorial is an overview of the process of ATP production, which includes glycolysis, the TCA cycle, beta-oxidation and the electron transport chain
This tutorial is the second in the Cellular Respiration series. This tutorial provides a complete description of Glycolysis, including all the enzymes involved and chemical structures for each step
This tutorial is the third in the Cellular Respiration series. This tutorial provides an overview of the TCA Cycle (also known as the Krebs Cycle) with a particular focus on the carbon balance throughout the cycle and the enzymes involved in each step.
This tutorial is the fourth in the Cellular Respiration series. This tutorial provides an overview of Beta-Oxidation, including activation and transport of fatty acids into the mitochondria
This tutorial is the fifth in the Cellular Respiration series. This tutorial provides an overview of Oxidative Phosphorylation, in particular the Electron Transport Chain.